Back to: O level chemistry notes full Uganda syllabus
An acid is a compound which when dissolved in water produces hydrogen ions (H+) as the only positively charged ion. There are basically two types of acids; mineral and organic acids.
a) Mineral acids
These are called mineral acds because they ar derived from minerals, examples include, the common laboratory acids like sulphuric acid (H2SO4), hydrochloric acid (HCl), nitric acid (HNO3) and phosphoric acid(H3PO4).
b) Organic acids
These are acids derived from organic compounds and examples include: ethanoic acid (CH3COOOH) found in vines, citric acid found in fruits and lactic acid found in milk.
Properties of acids
- Acids have a sharp sour taste.
- Acids change colors of indicators e.g. turn blue litmus paper red.
- Acids ionize in water to produce hydrogen ions (H+).

- Most acids are corrosive (i.e. have burning effects) and poisonous
- Dilute acids react with reactive metals to produce hydrogen gas and a salt.
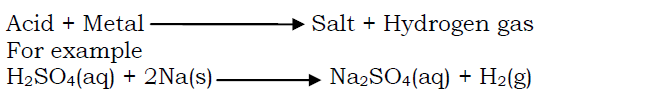
Acids react with carbonates and hydrogen carbonates to liberate carbondioxide gas. When an acid reacts with a carbonate,a salt, water and carbondioxide gas are produced.
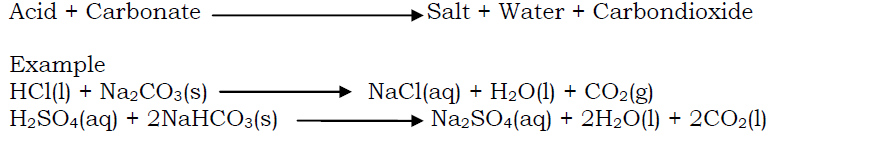
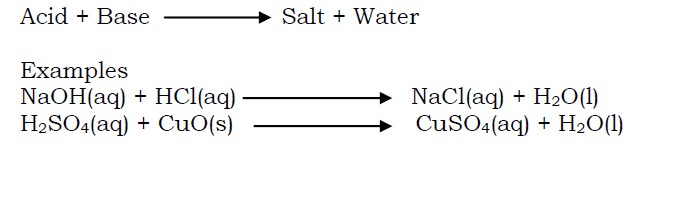
Acids react with bases to to form salt and water
Preparation of acids
- By the reaction between an acid anhydride (an oxide of a non metal) and water.
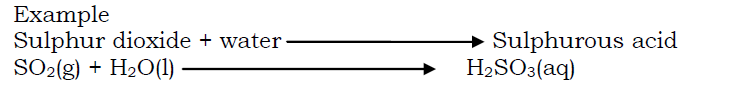
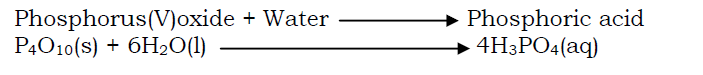
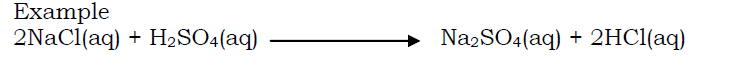
By displacing a weaker acid (more volatile acid) from its salt by a stronger acid (less volatile acid).
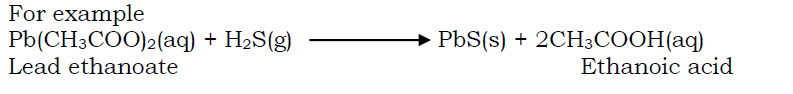
By precipitating an insoluble sulphide from a metallic salt by hydrogen sulphide.
Basicity of an acid
This is the number of hydrogen ions that can be produced when one molecule of an acid ionizes in water. Acids can be categorized interms of basicity as:
a) Monobasic acid
Strength of acids
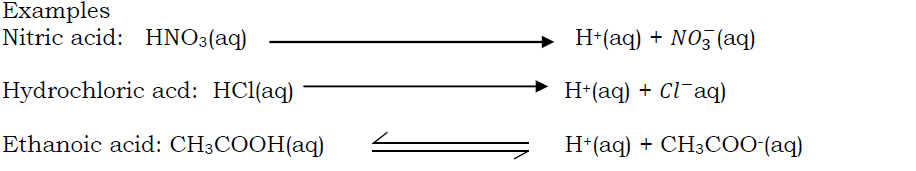
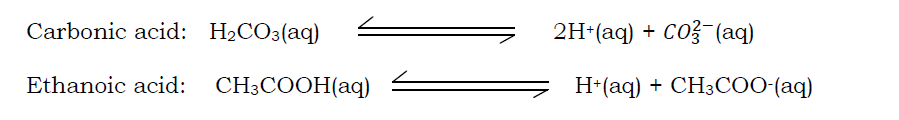
Strength of an acid refers to the ease with which an acid ionize to produce hydrogen ions. According to strength, acids can be categorized as strong or weak.
- Strong acid: Is an acid that dissociates completely into ions when dissolved in water (it ionizes completely in water). Examples include:

Weak acid: Is an acid that does not completely ionize in water. Some of the acid molecules do not ionize, examples include:
Uses of acids
i) Acids are used in car batteries
ii) Sulphuric acid is used in the manufacture of soap, detergents and paints. Plastics e.t.c.
iii) Ethanoic acid is used in the preservation of fruits and vegetables.
iv) Hydrochloric acid in the stomach of human being helps in the digestion of food.
v) Acids are used in the manufacture of fertilizers e.g. sulphuric acid.
vi) Ascorbic acid in fruits are useful to our bodies.
vii) Nitric acids are used in the manufacture of explosives.
Bases and Alkalis
A base is a substance which reacts with an acid to form salt and water only.
Bases are mainly oxides of metals like: copper(II)oxide, CuO; zinc oxide, ZnO;calcium oxide, CaO; magnesium oxide, MgO;or hydroxides of metals and ammonium groups like sodium hydroxide, NaOH; potassium hydroxide, KOH; calcium hydroxide,Ca(OH)2 and ammonium hydroxide, NH4OH.
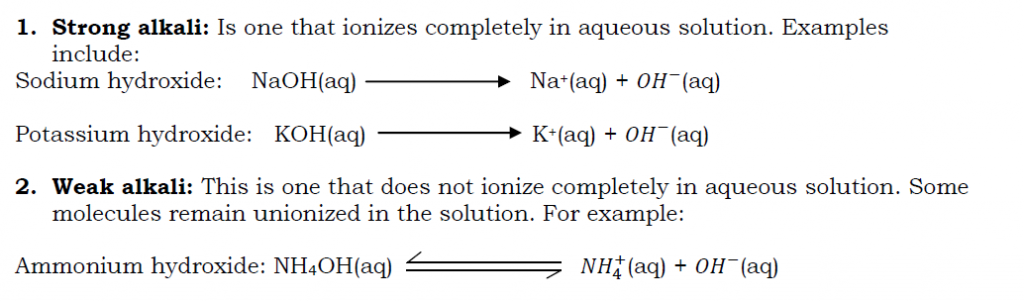
Bases that are soluble in water are known as alkalis.
Alkalis
An alkali is a base that dissolves in water to produce hydroxide ions ( ) as the only negatively charged ion. A solution of a base in water is called an alkaline solution.
Examples of alkalis include: sodium hydroxide or caustic soda( NaOH), potassium hydroxide or caustic potash (KOH), calcium hydroxide or lime water (Ca(OH)2) and ammonium hydroxide or aqueous ammonia (NH4OH).
Properties of alkalis
- Alkalis have a bitter taste
- Alkalis are soapy and feel slippery
- Alkalis turn red litmus paper blue
- Alkalis react with acids to produce salt and water.

5.Alkalis react with ammonium salts to produce ammonia gas. E.g.

6. Alkalis precipiatate insoluble metal hydroxides from solutions of their salts. E.g.

Strength of bases/ alkalis
This is the ease with which a base/ alkali dissociate or ionize. The bases/ alkalis can be categorized as strong or weak.
Neutralization reaction
This is the reaction between an acid and a base to produce salt and water only. The resulting solution is neutral (i.e neither acidic nor alkaline). Examples of neutralization reactions are:
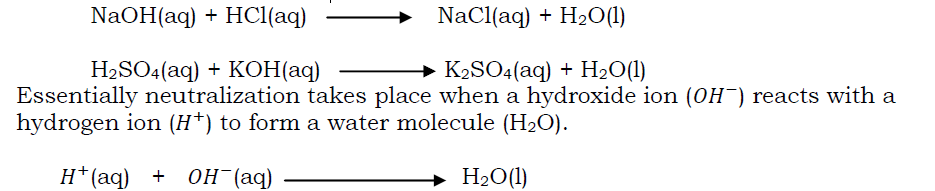
INDICATORS (ACID-BASE INDICATORS)
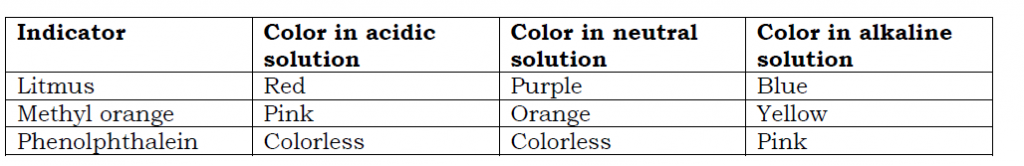
An acid-base indicator is a substance that shows different colors in acidic and alkaline solutions. Indicators are used to establish substances that are acidic, alkaline or neutral. The color change of the indicator depends on the strength of the acid or the base/ alkaline.
Some examples of indicators used in chemistry experiments include: Litmus paper; Methyl orange; Bromothymol blue; Bromothymol red; Phenolphthalein; Universal indicators e.t.c
Color changes of some indicators in acidic, alkaline and neutral solutions are shown below
Plant extract as a simple acid-base indicators
Preparation of indicators from flowers
Procedure
Collect a handful of flowers with brightly colored petals such as hibiscus and morning glory.
NB Use only one type of flowers and do not mix them
Remove the petals from the flowers, put them in a motor and crush them carefully
Add a little ethanol to the crushed petals and stir with a pestle
Carefully decant the mixture and keep the colored solution in a test tube. This acts as an indicator. Take note of the color of the indicator
Universal indicator
A universal indicator is a mixture of other simple indicators and under goes a range of color changes in solution of different PH. A universal indicator shows the extent of alkalinity or acidity (i.e in addition to showing whether a solution is acidic or alkaline, it shows whether the acid is weak or strong.) The indicator may be available in solution or paper form