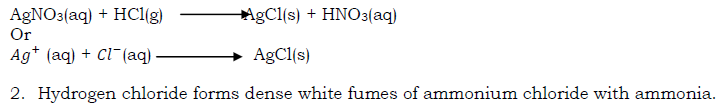Back to: O level chemistry notes full Uganda syllabus
The four non metals fluorine, chlorine, bromine and iodine make up a family of related elements called halogens meaning salt producers since they react with most metals to form electrovalent salt like compounds.
Preparation of chlorine
Chlorine can be generally prepared by the removal of hydrogen from hydrochloric acid i.e. oxidation of hydrochloric acid. This can be done by a substance containing oxygen (an oxidizing agent) that will combine with the hydrogen to form water. The oxygen for the oxidation of hydrochloric acid is provided by any of the following:
Potassium manganate(VII) or potassium permanganate (KMnO4)
i) Manganese(IV) oxide (MnO2)
ii) Lead(II) oxide (PbO2)
iii) Tri lead tetraoxide (Pb3O4)
The common oxidizing agents used in the preparation of chlorine are potassium permanganate and manganese(IV) oxide.
Preparation of chlorine from hydrochloric acid by oxidation with manganese(IV) oxide
Procedure
- Place some manganese(IV) oxide into a flask. Lumps are preferably used as the powder is very reactive
- Fit the apparatus as shown below
- Pour concentrated hydrochloric acid down the thistle funnel
- Heat the mixture in the flask

Observation
Effervescence occurs evolving a greenish yellow gas (chlorine gas). Chlorine is evolved together with a small amount of hydrogen chloride gas (misty fumes) which is removed by passing it through the first bottle containing water.
The gas is then dried using concentrated sulphuric acid and collected by down ward delivery method since it is denser than air.
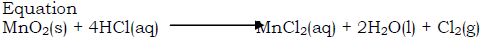
Chlorine being a poisonous gas, it is prepared in the fume cupboard.
Preparation of chlorine from hydrochloric acid by oxidation with potassium permanganate
Procedure
- Place solid potassium permanganate in a flask
- Fit the apparatus as shown below
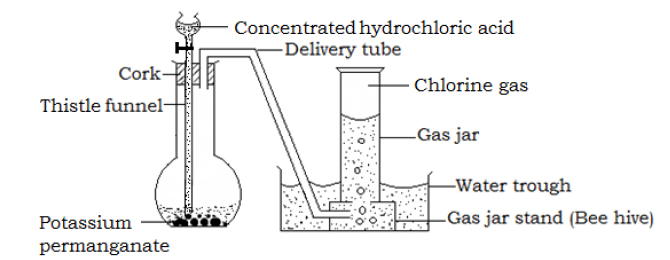
Drop on the potassium permanganate concentrated hydrochloric acid from the tap funnel. As each drop reaches the Manganese(IV) oxide, a corresponding quantity of chlorine, a greenish yellow gas is evolved.
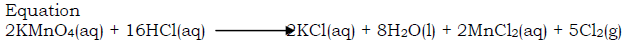
The gas collection is by down ward deliver of over brine of hot water of in a gas syringe.
This is the most convenient laboratory method because of the following reasons:
- It does not require heating
- Rate of production of chlorine can be regulated
- If the gas is collected over brine, the experiment can be done out of the fume chamber
Chlorine can be prepared from sodium chloride by adding concentrated sulphuric acid to an intimate mixture of sodium chloride and Manganese(IV) oxide. Traces of hydrogen chloride produced are removed by passing the gas over water. The gas is dried and collected by down ward delivery method.
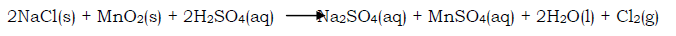
Chlorine can also be prepared from bleaching powder. The bleaching powder is placed in a flask and a dilute acid e.g. nitric acid, hydrochloric acid is added to the powder. Effervescence occurs as a greenish yellow gas (chlorine gas) is evolved. The gas is then dried and collected by any of the methods discussed.
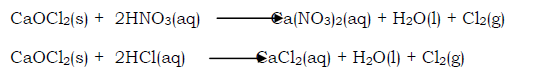
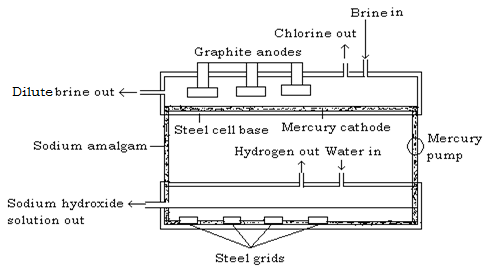
Industrial manufacture of chlorine
Chlorine is produced commercially by electrolysis of sodium chloride (brine) solution. Chlorine is evolved at the anode of a specially designed cell, and since the other electrode product (sodium hydroxide, at the cathode) reacts with chlorine, they must be kept apart. This is effected by a circulating mercury diaphragm.
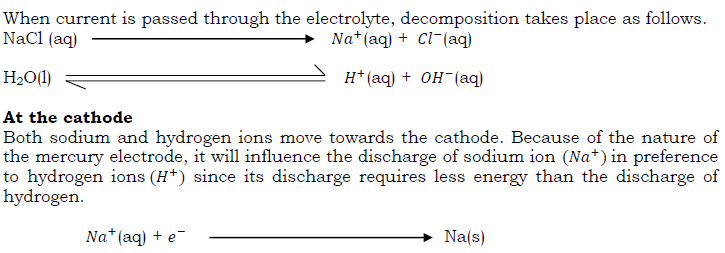
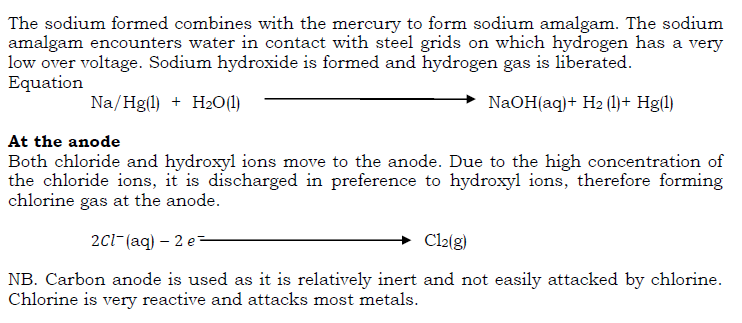
Properties of chlorine
Physical properties
- It is a greenish yellow gas with a chocking, unpleasant, irritating smell.
- It is slightly soluble in water forming a yellowish chlorine water which is a mixture of hydrochloric acid and hypochlorous acid.

It is denser than air.
- It turns damp blue litmus paper red the bleaches it. Its bleaching action is due to the formation of hypochlorous acid. Dry chlorine does not bleach.
- Dry Chlorine does not bleach and extinguishes a burning splint.
Test for chlorine:
It is a greenish yellow gas which turns moist blue litmus paper red then bleaches it. This is because, the gas is acidic.
Chemical properties
- Chlorine as a bleaching agent
Pour a little litmus solution into a gas jar of chlorine.
Observation: The litmus immediately turns colourless.
Chlorine bleaches colour from most dyes and will remove colour from writing ink (but not printer‘s ink, which consists mainly of carbon which chlorine does not attack.)
The bleaching action
Chlorine reacts with water to form hypochlorous acid

The hypochlorous acid is a very reactive compound and readily gives up its oxygen to the dye, to form a colourless compound.
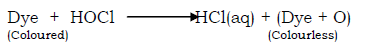
The hypochlorous acid turns the coloured dye to colourless by oxidation reaction since it gives up its oxygen to the coloured dye.
This indicates that dry chlorine will not bleach since there will be no hypochlorous acid formed.
Hypochlorous acid is also used to kill bacteria and germs in drinking water, swimming pools and in sewage treatment.
- Effect of sunlight on chlorine water
Pass chlorine gas into water in a beaker until water becomes yellow green in colour i.e. chlorine water is formed. Fill a long tube with this chlorine water and invert it in a beaker containing some of the water and expose it to bright sunlight.
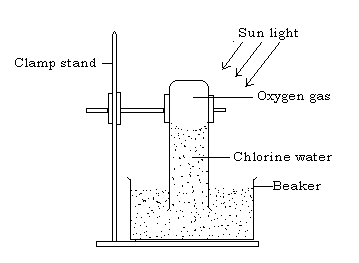
After some times, a gas collects in the tube and on applying a glowing splint, the gas rekindled it showing that the gas is oxygen.
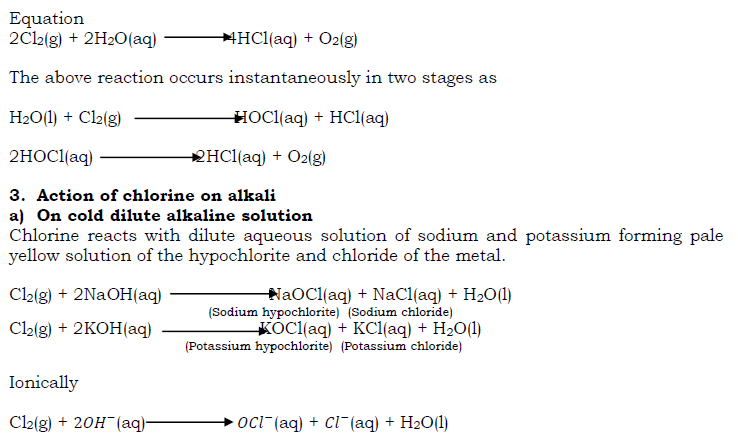
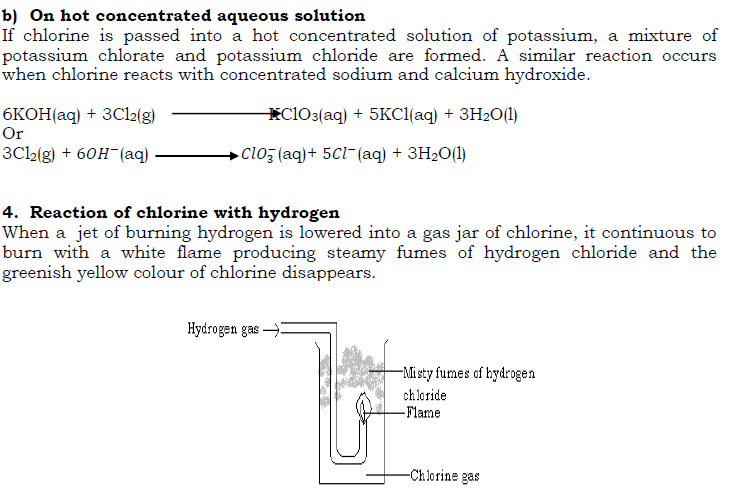

A mixture of hydrogen and chlorine also explodes when exposed to bright sunlight. This shows the great affinity of chlorine for hydrogen. The reaction is slow in dim sun light and reaction does not take place in the absence of light.
- Chlorine as an oxidizing agent
An oxidizing agent is one which can
i) Remove hydrogen from a compound
ii) Accept electrons donated by metals
Chlorine removes hydrogen from many compounds as well as accepts electrons from metals. The oxidizing property of chlorine is illustrated by the following reactions.
i) Reaction with hydrogen sulphide
When a gas jar of hydrogen sulphide is inverted over a gas jar of chlorine, yellow solids of sulphur and white fumes of hydrogen chloride are formed.

ii) Reaction with ammonia
Ammonia burns in chlorine and is oxidized to nitrogen and chlorine itself is reduced to hydrogen chloride.

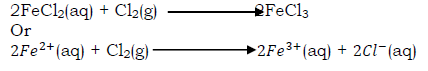
iii) Oxidation of iron(II) to iron(III)
Chlorine accepts electrons like other oxidizing agents and is converted to chlorine ion.

When chlorine gas is bubbled through a pale green solution of iron(II) chloride, it changes to yellow due to the formation of iron(III) chloride. When sodium hydroxide is added to this solution it forms red brown precipitates. This indicates that iron(II) chloride has been oxidized to iron(III) chloride.
iv) Reaction of chlorine with hydrocarbons e.g. turpentine (C10H16)
Hydrocarbons consist of only carbon and hydrogen. Chlorine removes hydrogen from hydrocarbon to form hydrogen chloride and black carbon particles are left.
Procedure
Warm a little turpentine in a dish. Dip into it a filter paper and drop the filter paper with turpentine in a gas jar containing chlorine
Observation
A red flash accompanied by a violent reaction occurs. Black cloud of solid particles of carbon is produced and hydrogen chloride gas is also formed.

The presence of hydrogen chloride can be shown by blowing the fumes from ammonia bottle across the top of the jar. Dense white fumes of ammonium chloride are formed.

6) Displacement reaction of chlorine
Chlorine being more reactive than the other halogens displaces them from their salts.
i) Reaction of chlorine with potassium bromide
When chlorine is bubbled through a saturated solution of potassium bromide, the clear solution immediately turns red (due to formation of bromine water) and a drop of a red liquid (bromine) is observed at the bottom of the boiling tube.
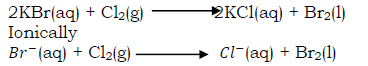
Bromine dissolves in tetra chloromethane to form a reddish brown solution.
ii) Displacement of iodine
When chlorine is bubble through a solution of potassium iodide, the clear solution turns to the characteristic dark brown ―iodine‖ colour and a black solid (iodine) is deposited as iodine is only slightly soluble in water. On warming the solution, the characteristic violet vapour of iodine is seen.
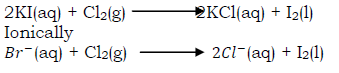
Iodine dissolves in tetra chloromethane to form a violet solution.
7) Reaction of chlorine with non metals
i) Phosphorus
When a piece of dry yellow phosphorus is lowered in a gas jar of chlorine, it burns spontaneously giving off white fumes of chlorides of phosphorus mainly phosphorus tri chloride (PCl3)
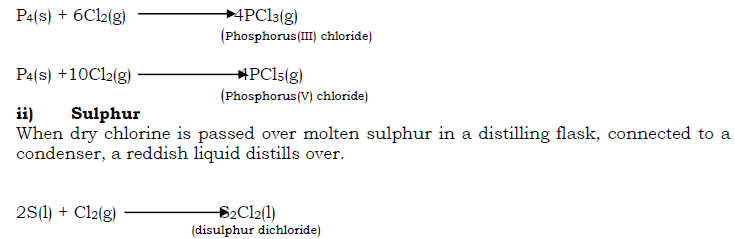
8) Reaction of chlorine with metals
i) Reaction with sodium and magnesium
Burning sodium and magnesium continue to burn is chlorine forming white fumes of sodium and magnesium chlorides respectively.

ii) Reaction with Dutch metal
Dutch metal is an alloy of copper and zinc. When a piece of Dutch metal is dropped in a gas jar of chlorine and heated, it burns with a green flame to form copper(II) chloride and Zinc chloride.

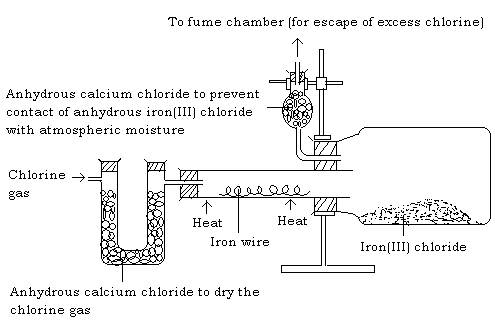
iii) Action of chlorine on iron (preparation of iron(III) chloride by direct synthesis)
Procedure
- Place a coil of iron wire in a hard glass tube in the apparatus below. The iron coil must be free of rust.
- Pass a stream of dry chlorine over it.
- Heat the wire and stop the heating the moment the reaction starts.
Observation
The wire glows and the reaction continues without application of heat indicating that the reaction is exothermic. Black crystals of iron(III) chloride sublimes and collects in the small bottle which acts as a condenser.

Formation of iron(III) chloride shows that chlorine is an oxidizing agent. The iron (II) chloride formed is immediately oxidized to iron(III) chloride.
The black crystals of anhydrous iron(III) chloride should be placed in a desiccator as they are very deliquescent.
Note
- Sodium chloride can be made in a similar way.
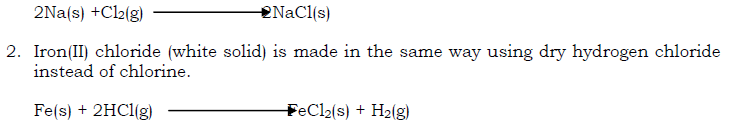
Uses of chlorine
- Chlorine is extensively used as a bleaching agent and in the manufacture of bleaching agents.
- It is used for making domestic antiseptic solutions such as sodium hypochlorite.
- Chlorine is used in the manufacture of chlorates used for example as weed killers.
- Manufacture of hydrogen chloride which is used in the manufacture of plastic like PVC (polyvinylchloride).
- Manufacture of many organic chemicals e.g. tetra chloromethane (CCl4), 1,1,2-trichloroethene (C2HCl3). These compounds are solvents used to remove grease from other substances (degreasing agents), dry cleaning fluids.
- Chlorine is used to sterilize water for domestic and industrial uses i.e. it kills bacteria and other germs in water and is used in water purification process.
HYDROGEN CHLORIDE
Laboratory preparation
Hydrogen chloride can be prepared in the laboratory by the action of concentrated sulphuric acid on common salt (rock salt).
Procedure
- Place sodium chloride in flask and fit the apparatus as shown below.
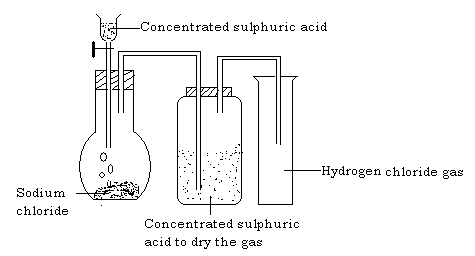
- Add concentrated sulphuric acid down the funnel.
Effervescence occurs and misty fumes are observed. The gas is passed through a wash bottle containing concentrated sulphuric acid to dry the gas and it is collected by down ward delivery method as it is denser than air.

Note
- Sodium chloride is used because it is cheap and readily available.
- The reaction proceeds in the cold though a further yield is obtained in the industrial process by heating.
- The sulphate is not obtained under laboratory conditions because its formation requires a higher temperature but is obtained during the industrial process.
Test for hydrogen chloride - When the gas is bubbled through a solution of silver nitrate and nitric acid, it forms white precipitates of silver chloride.