Back to: O level chemistry notes full Uganda syllabus
Hydrogen is the smallest element and the lightest gas. Hydrogen usually does not occur is Free State but in combined states as water, acid, hydrocarbons and other organic compounds.
Laboratory preparation of hydrogen
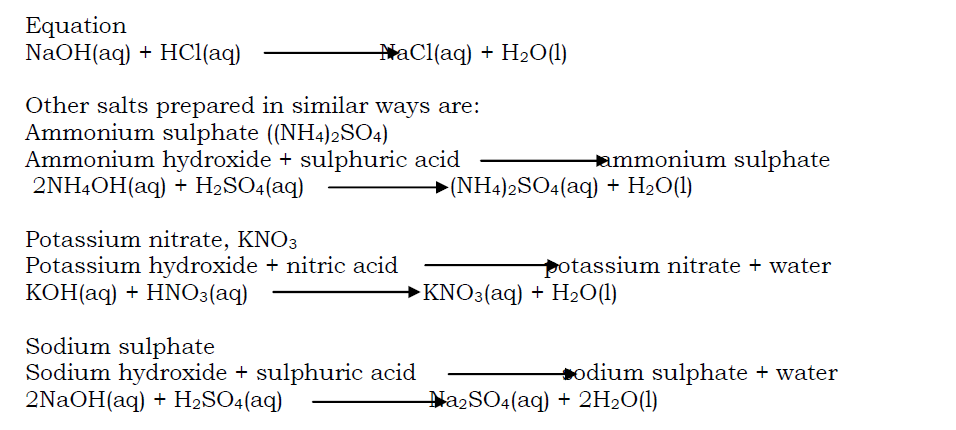
Hydrogen is prepared in the laboratory by the action of either dilute hydrochloric acid or dilute sulphuric acid on zinc granules or zinc metal. (zinc granule are used instead of pure z
Set up
Place some zinc granules in the flask and add to it a little copper (II) sulphate solution. The copper (II) sulphate acts as a catalyst in the preparation of hydrogen gas.
Arrange the apparatus as shown above and add dilute sulphuric acid/ hydrochloric acid to the zinc granules through the funnel.
Effervescence occurs as hydrogen gas is produced. The gas is then collected over water. However, if the gas is required dry, it is passed through a wash bottle
containing concentrated sulphuric acid and collected by upward delivery method or the gas is passed through a U-tube containing fused calcium chloride to dry the gas.
Equation

Test for hydrogen
When a burning splint is brought into a gas jar of hydrogen, the splint will be extinguished with a ―pop‖ sound.
Properties of hydrogen
a) Physical properties
- It is the lightest gas known (lighter than air)
- It is colorless, odourless and tasteless
- It is slightly soluble in water
- It is a neutral gas (has no effects on indicators)
b) Chemical properties
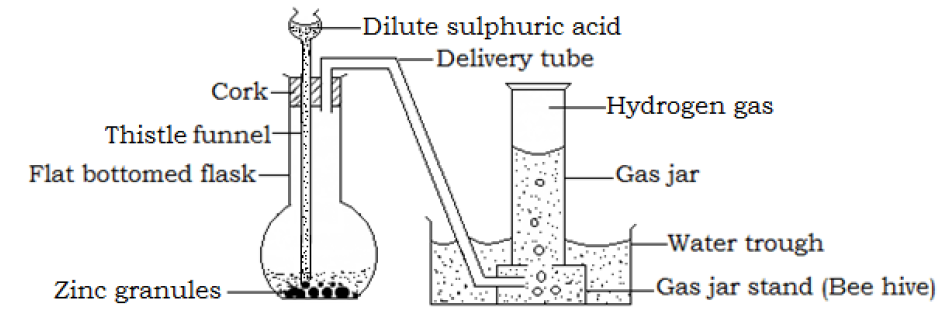
- Combustion (burning) of hydrogen
Hydrogen burns in air with a faint blue flame to produce water vapor. The gaseous product can be condensed in a cool environment to form a colorless liquid the turns white anhydrous copper (II) sulphate to blue indicating that it is water.
- Reduction action of hydrogen
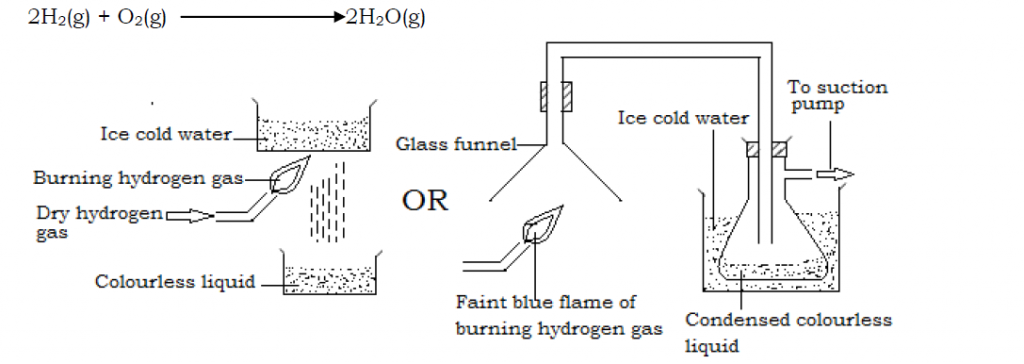
Hydrogen is a reducing agent. It removes oxygen from the oxides of some metals (less reactive metals) like lead and copper forming the metal and the hydrogen gas itself is oxidized to water.
Reduction of copper(II)oxide
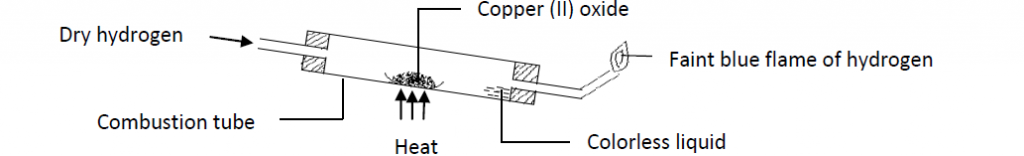
When dry hydrogen gas is passed over heated copper (II) oxide in a combustion tube, a red glow spreads through the copper (II) oxide and the oxide turns from black to brown as the oxide is reduced to copper metal (brown in color). The hydrogen itself is oxidized to water which collects as a colorless liquid.

Hydrogen also reduces lead (II) oxide and triirontetraoxide to lead and iron respectively.
Precautions
- Dry hydrogen gas must be used.
- The combustion tube must be in a slanting position so as to prevent the water formed from running back to the hot part of the tube.
- Hydrogen should be passed through the combustion tube to expel out air before heating the oxide.
- Hydrogen should be passed through the tube for some times after heating .This prevents re oxidation of the copper.
- The excess hydrogen should be burnt to prevent explosion with air.
Reduction is the addition of hydrogen to a substance or the removal of oxygen from a substance.
Oxidation is the removal of hydrogen from a substance or the addition of oxygen to a substance. - Reaction with chlorine
A mixture of chlorine and hydrogen appears not to react at room temperature, but when exposed to sun light or heated, the mixture explodes forming misty fumes of hydrogen chloride gas.

- Reaction with reactive metals
Hydrogen reacts with highly reactive metals to form hydrides. For example, sodium reacts with hydrogen to form sodium hydride.

Uses of hydrogen
- Hydrogen is a very light gas and therefore used to fill balloons.
- Hydrogen with oxygen form oxy-hydrogen flame which is very hot and used for welding and cutting metals.
- Hydrogen is used in the manufacture of ammonia by Haber process.
- Hydrogenation of vegetable oil makes it hard and used for making margarine and cooling fats like blue band and kimbo.
- It is used in the manufacture of hydrogen bombs.
- Hydrogen is also used as fuel for rockets.
Sample questions on water and hydrogen
- Briefly describe the water cycle. Mention the major sources of water. How is waater beneficial to you and to the community from where you come?
- What is water pollution? What are the main sources of water pollution? How do the pollutants mainly reach the water bodies?. Describe briefly the effects of water pollution to living organisms in water bodies.
- In an attempt to make water available for domestic use, water is treated. Explain in detail the steps taken in water purification processes.
- Describe the reactions of sodium, potassium, magnesium and calcium with water stating clearly the conditions under which the reactions take place.
- What is meant by soft and hard water? Give examples of each. Explain the types of hard water that you know giving the causes of each; outline the physical and chemical methods of softening each type of hardness. (Where appropriate use equations to illustrate). Mention the advantages and disadvantages of hard water.
- Describe the aid of a labeled drawing how hydrogen gas is prepared in the laboratory. How do you confirm that the gas produced in your description is hydrogen gas?
- How do you show that water is an oxide of hydrogen? Describe the reduction reaction of hydrogen using of copper(II) oxide (What are the precautions for the reaction). Outline at least five uses of hydrogen gas.
SALTS
A salt is a compound formed when either all of part of the ionisable hydrogen of the acid is replaced by a metallic ion or ammonium ion. Or
A salt is an ionic compound consisting of a positive metallic or ammonium ion and a negative ion derived from an acid.
Salts get their names from the acids they are derived from. Examples are in the table below.
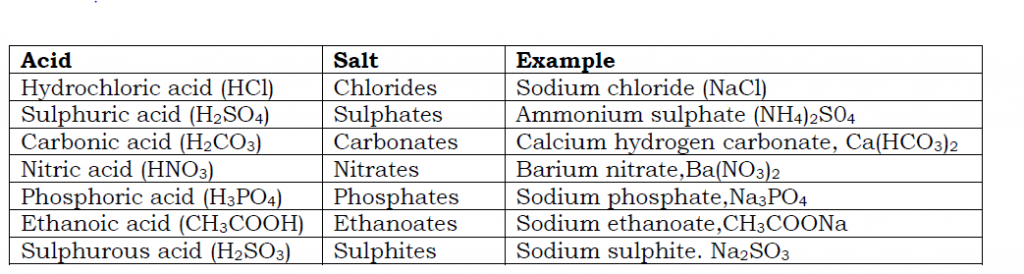
Types of salts
a) Normal salt
This is a salt produced when all the ionisable hydrogen of the acid is replaced by a metallic or ammonium ion. These salts do not contain ionisable hydrogen. Examples include; sodium chloride, NaCl; ammonium nitrate, NH4NO3; Magnesium sulphate, MgSO4; lead (II) bromide, MgBr2 and sodium phosphate, Na3PO4.
All normal salts have PH of 7 except salts formed from
i) Strong bases and weak acids e.g. sodium carbonate (Na2CO3) and potassium ehanoate (CH3COOK). These salts in solution have PH value more than 7.
ii) Strong acids and weak bases e.g. ammonium chloride (NH4Cl). The salts have a PH value less than 7 in solution.
b) Acid salts
An acid salt is a salt formed when only part of the ionisable hydrogen of the acid is replaced by ammonium or metallic ion. These salts contain ionisable hydrogen and examples include: sodium hydrogensulphate, NaHSO4; calcium hydrogencarbonate, Ca(HCO3)2; and potassium hydrogen carbonate, KHCO3.

c) Basic salts
Basic salt are formed when insufficient acid is present to neutralize the available base. E.g. basic zinc chloride (Zn(OH)Cl) and basic magnesium chloride (Mg(OH)Cl).
All monobasic acids form normal salts while dibasic and tribasic acids form both normal and acid salts.
Naming of salts
Salts are named by adding the name of the radical or ion of the acid after the name of the metal or ammonium. Examples are:

SOLUBILITY OF SALTS
Solubility is the amount of solute in grams required to saturate 100g of solvent (water) at a particular temperature.
A salt is described as soluble if it can dissolve in a given solvent and insoluble if it cannot dissolve in the solvent. Salts have varying degree of solubility in water as described below:
All ammonium, sodium, and potassium salts are soluble in water.
All nitrate salts are soluble in water.
All chloride salts are soluble in water except silver chloride, lead (II) chloride (sparingly soluble) and mercury (I) chloride.
All sulphate salts are soluble in water except lead (II) sulphate and barium sulphate. Calcium sulphate is sparingly soluble in water.
All carbonate salts are insoluble in water except sodium, potassium and ammonium carbonates.
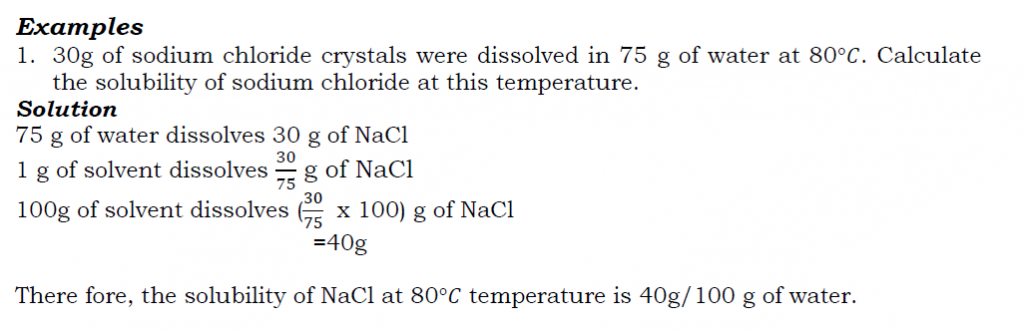
Determining the solubility of a salt e.g. sodium chloride
Procedure
- Take about 50cm3 of distilled water in a beaker.
- Add sodium chloride crystals to the water a little at a time while stirring continuously until when no more salt dissolves. The solution formed is saturated.
- Weigh a clean evaporating dish and pour into it a little of the clear salt solution.
- Weigh the evaporating dish with the salt solution and evaporate the solution to dryness carefully through a water bath.
- Allow the evaporating dish to cool and reweigh the dish with the dry salt.
Results
Mass of empty dish= a g
Mass of dish + saturated solution = b g
Mass of dish + dry salt = c g
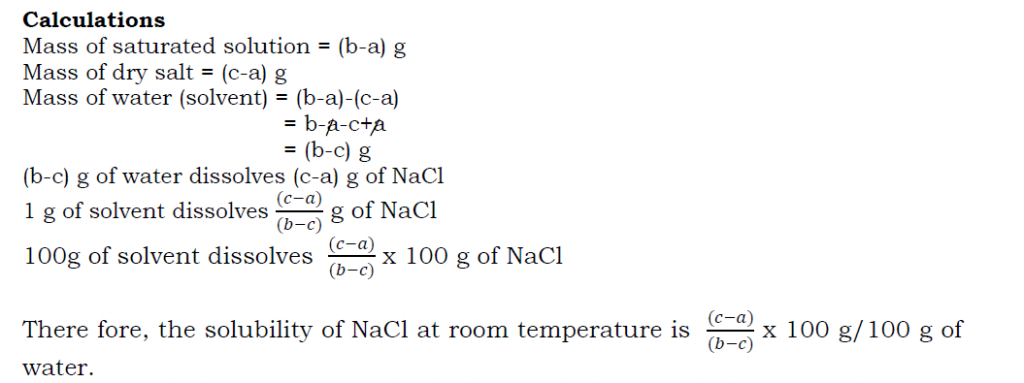


Exercise
- In an experiment to determine the solubility of potassium nitrate at 20˚C, the following results were obtained.
Mass of evaporating dish + saturated solution = 100.7g
Mass of evaporating dish = 65.3g
Mass of dish + dry salt = 73.8 g
Use the data above to calculate the solubility of potassium nitrate at 20˚C. Clearly show your working.
Factors that affect the rate of solubility of salts - Amount of solvent
Solubility of most salts increase with increase in the amount of solvent used.
- Nature of solvent/solute
Solubility of a salt may increase or decrease depending on the nature of solvent or solute. - Temperature
Solubility of most salts increase with increase in temperature. For example, potassium chlorate and potassium nitrate. Solubility of a few salts like calcium chloride and calcium sulphate decrease with increase in temperature. The solubility of sodium hydroxide and gases as well also decrease with increase in temperature.
Solubility curve
A solubility curve is a graph that shows how the solubility of a salt varies with temperature. The graph is obtained by plotting solubility (on the vertical axis) against temperature (on the horizontal axis).
Solubility curve of some common salts

The solubility of potassium chloride, potassium nitrate and potassium chlorate increase with increase in temperature. The solubility of potassium nitrate increases most rapidly, followed by potassium chlorate then potassium chloride.
The solubility of sodium chloride increases very slightly with increase in temperature.
The solubility of calcium sulphate decreases with increase in temperature.
Uses of solubility curves
- It can be used to find the solubility of a salt at a given temperature.
- It gives the temperature at which a given amount of salt saturates 100g of solvent.
- It can be used to explain the trend of solubility of salts.
- A solubility curve can be used to calculate the mass of salt obtained by cooling a solution from a higher temperature to a lower temperature.
Mass of salt= (solubility at a higher temperature – solubility at a lower temperature)
For example, if a salt P with solubility of 180g/100g of water at 90˚C was cooled to a temperature of 30˚C where its solubility is 25g/100g of water. Calculate the mass of salt formed formed after cooling the solution.
Solution

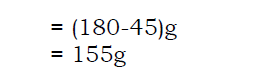
Application of solubility
- Solubility is used to separate soluble salts from a mixture by fractional crystallization.
- It is used in the extraction of salts from large water bodies like lakes and seas.
Exercise - a) Describe an experiment that you would carryout to determine the solubility of potassium nitrate at 15˚C.
b) Determine the solubility in water of substance S at room temperature from the following data.
Mass of evaporating basin 25g
Mass of evaporating basin + Saturated solution of S 55g
Mass of evaporating basin + Solid S 30g - a) Define the term solubility?
b) The table below shows the solubility (ies) of salt P in water at different temperatures.

i) plot a graph of solubility of P against temperature
ii) use your graph to determine
a) solubilities of P at 25˚C and 45˚C
b) the mass of crystals deposited when a solution of P is cooled from 50˚C to 25˚C
iii) calculate the mass of P that would dissolve in 45g of water at 25˚C
PREPARATION OF SALTS
The method of salt preparation depends on whether the salt is soluble in water or not. Soluble salts are prepared by crystallization and neutralization. Insoluble salts are prepared by precipitation or double decomposition. Other salts are prepared by direct synthesis.
Preparation of soluble salts
Soluble salts are prepared using dilute acids and metals, metals oxides, metal hydroxides and metal carbonates.
General procedure
- Place some dilute acid in a beaker
- Warm the acid and add the metal, metal oxide, metal hydroxide and metal carbonate bit by bit until in excess to ensure that the acid is completely used up.
- Filter the excess metal, metal oxide, metal hydroxide or metal carbonate and collect the filtrate.
- Saturate the filtrate by evaporating and allow the solution to cool as it cools to form the salt crystals.
- Filter the crystals and wash them with water.
- Dry the crystals in an oven, or under sun shine or between filter papers.
- Preparation of salts from metals and dilute acids
Salts prepared by this method are soluble salts of iron, magnesium, aluminium and zinc. (I.e. metals higher than lead and lower than calcium in the reactivity series.)
N.B. Nitrates cannot be prepared using this method because dilute nitric acid being an oxidizing agent, does not react with metal to liberate hydrogen gas.
Example
Laboratory preparation of zinc sulphate crystals from zinc metal/powder
- Put dilute sulphuric acid in a beaker and heat it gently until when it‘s hot.
- Add zinc powder to the hot acid bit by bit while stirring until when the zinc powder is in excess.
- Filter off the excess zinc powder to obtain zinc sulphate solution as the filtrate.
- Saturate the filtrate by evaporating.
- Allow it to cool and form crystals of the salt.
- Filter the crystals and wash them with distilled water.
- Dry the crystals either in qaan oven of under the sun or between filter papers.
Equation


- Preparation of salts from metal oxides and dilute acids
Example
Preparation of copper (II) sulphate from copper (II) oxide in the laboratory
- Put dilute sulphuric acid in a beaker and heat it gently until when it‘s hot.
- Add copper (II) oxide to the hot acid bit by bit while stirring until when the copper (II) oxide is in excess.
- Filter off the excess copper (II) oxide to obtain copper sulphate solution as the filtrate.
- Saturate the filtrate by evaporating.
- Allow it to cool and form crystals of the salt.
- Filter the crystals and wash them with distilled water.
- Dry the crystals either in an oven or under sunshine or between filter papers.
Equation

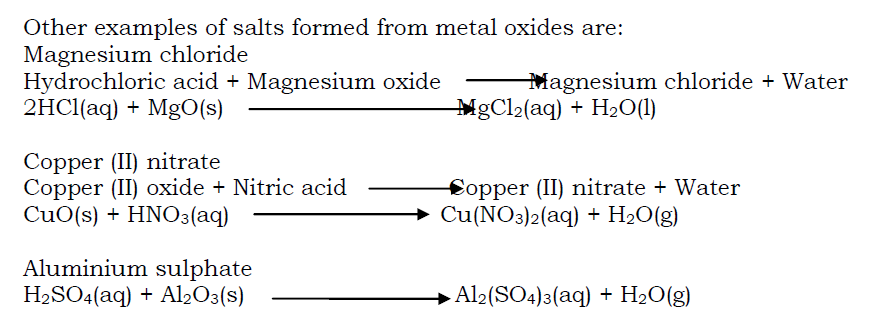
- Preparation of salts from insoluble metal carbonates
Example
Preparation of lead (II) nitrate from lead (II) carbonate
- Pour dilute nitric acid in a beaker and warm it gently.
- Add lead (II) carbonate a little at a time. Effervescence occurs as carbondioixde is evolved.
- Continue adding the carbonate until when it is in excess and no more effervescence occurs.
- Filter off the excess carbonate to get a colourless filtrate.
- Evaporates the filtrate by heating gently to obtain a saturated solution.
- Cool the saturated solution to obtain white crystals of lead (II) nitrate salts.
- Wash the crystals with cold distilled water and dry either on sun shine, in an oven or between filter papers.
- Preparation of salts from metal hydroxides
Example
Preparation of lead (II) nitrate starting from lead (II) hydroxide
- Pour dilute nitric acid in a beaker and warm it gently.
- Add lead (II) hydroxide a little at a time while until when it is in excess.
- Filter off the excess hydroxide to get a colorless filtrate.
- Evaporates the filtrate by heating gently to obtain a saturated solution.
- Cool the saturated solution to obtain white crystals of lead (II) nitrate salts.

Laboratory preparations of salts whose carbonates, oxides and hydroxides sare insoluble
These salts include potassium, sodium and ammonium salts. The salts can be prepared by titration method (neutralization).
Neutralization is a reaction between an acid and a base to produce a salt and water only.
General procedure
- Put a known volume of hydroxide of a metal in a conical flask.
- Add 2 or 3 drops of an indicator.
- Run a suitable acid from the burette until when the color of the mixture just changes. Note and record the volume of acid used.
- Measure accurately the same volume of hydroxide as before and titrate with exactly the same volume of acid as recorded above.
NB. An indicator is not used in the second titration, since the volume of acid required to neutralize the fixed volume of base was already got. - Stir and heat the solution to make it saturated.
- Allow the hot saturated solution to cool as it forms salt crystals.
- The crystals are filtered off, washed with cold distilled water and dried in an oven, under sun shine or between filter papers.
Preparation of sodium chloride crystals in the laboratory
Procedure
- Put a known volume of sodium hydroxide in a conical flask.
- Add 2 or 3 drops of an indicator.
- Titrate the sodium hydroxide with hydrochloric acid from the burette until when the end point is reached (when the indicator changes color). Note and record the volume of acid used.
- Measure accurately the same volume of sodium hydroxide as before and titrate with exactly the same volume of hydrochloric acid as recorded above without using an indicator.
- Stir and heat the solution to make it saturated.
Allow the hot saturated solution to cool as it forms salt crystals.
- The crystals are filtered off, washed with cold distilled water and dried in an oven, under sun shine or between filter papers.