Back to: O level chemistry notes full Uganda syllabus
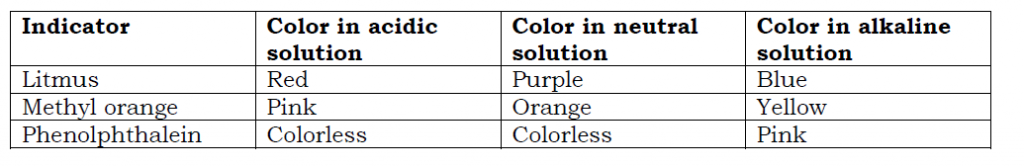
An acid-base indicator is a substance that shows different colors in acidic and alkaline solutions. Indicators are used to establish substances that are acidic, alkaline or neutral. The color change of the indicator depends on the strength of the acid or the base/ alkaline.
Some examples of indicators used in chemistry experiments include: Litmus paper; Methyl orange; Bromothymol blue; Bromothymol red; Phenolphthalein; Universal indicators e.t.c.
Color changes of some indicators in acidic, alkaline and neutral solutions are shown below
Plant extract as a simple acid-base indicators
Preparation of indicators from flowers
Procedure
Collect a handful of flowers with brightly colored petals such as hibiscus and morning glory.
NB Use only one type of flowers and do not mix them
Remove the petals from the flowers, put them in a motor and crush them carefully
Add a little ethanol to the crushed petals and stir with a pestle
Carefully decant the mixture and keep the colored solution in a test tube. This acts as an indicator. Take note of the color of the indicator
Universal indicator
A universal indicator is a mixture of other simple indicators and under goes a range of color changes in solution of different PH. A universal indicator shows the extent of alkalinity or acidity (i.e in addition to showing whether a solution is acidic or alkaline, it shows whether the acid is weak or strong.) The indicator may be available in solution or paper form.
PH and PH Scale
The PH of a solution is a number which shows the acidic or basic strength of the solution.
It can also be defined as the negative logarithm (to base ten) of hydrogen ion concentration. It is obtained by finding the negative logarithm of hydrogen ion concentration.
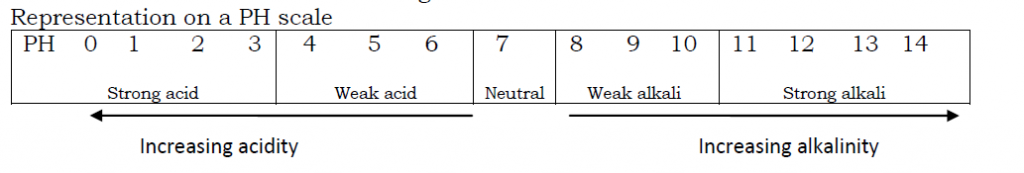
A PH scale measures the strength of an acid or a base/alkali. The PH scale runs from Zero (O) to fourteen (14).
The following are values on the PH scale for solutions of different PH.
An acidic solution has a PH less than 7
A neutral solutions has a PH of 7
An alkaline solution has a PH greater than 7
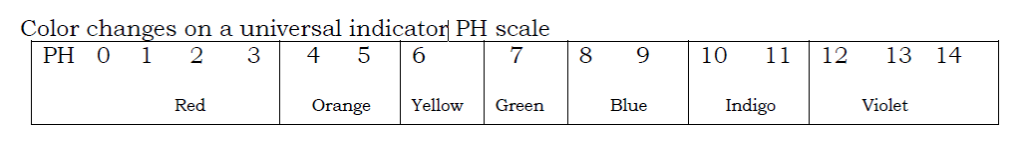
To find the PH of a solution using a solution of a universal indicator.
- Add some drops of the universal indicator to the solution being tested.
- Match the color change of the solution to the corresponding color on the universal indicator PH chart.
- Record the color change and the PH value.
CHEMICAL FAMILIES
Elements in the same group are referred to as chemical families because of the similarities in their chemical and physical properties. These elements in the same group have similar chemical properties because they have the same number of electrons in their outer most energy levels. The common chemical families are; alkaline metals; alkaline earth metals; halogens and noble gases.
Alkaline metals
These are elements of group I in the periodic table. These metals are very reactive and are kept under oil in the laboratory where they have no contact with water and air. They include lithium (Li), sodium (Na) and potassium (K). Atoms of all these elements each have a single electron on the outer most shell, which is easily lost during chemical reactions leaving a single positively charged ion. i.e.
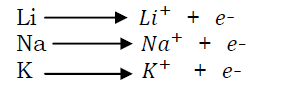
Physical properties
- They re harder than group I metals.
- They are good conductors of electricity and heat.
- They are silvery grey in color when freshly cut. However the shinny surface slowly tarnishes on exposure to air forming the metal oxide.
- Their melting and boiling points are higher than those of group I. This is because these metals release more electrons into the electron cloud forming a stronger metallic bond in the metal structure. This explains why they are also harder than group I metals.
Chemical reactions
Alkaline earth metals are more reactive than alkaline metals. This is because the valence electrons in alkaline earth metals are held more strongly due to increased effective nuclear charge making them not easily to be released. Reactivity of the metals increase down the group due to increase in atomic size.(calcium is the most reactive followed by magnesium then beryllium )
- Reaction with air
The metals burn in air with their characteristic flame forming oxides of the metal. Magnesium burns in air with a brilliant white flame forming white ash of magnesium oxide.
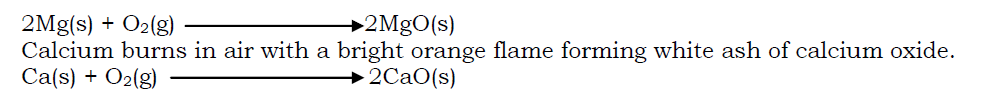

When the nitrides are dissolved in water and the solution warmed, ammonia gas is liberated and an alkaline solution is also formed.
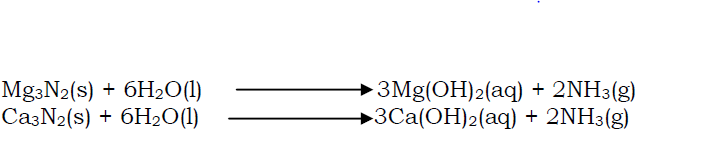
- Reaction with water
Beryllium does not react with water. Magnesium piece reacts very slowly with cold water producing small bubbles of hydrogen gas and an alkaline solution of magnesium hydroxide.

Magnesium reacts very rapidly with steam to produce white ash of magnesium oxide and hydrogen gas.

Calcium reacts steadily with water evolving hydrogen gas and calcium hydroxide.
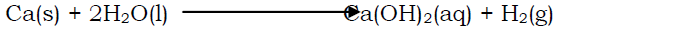
- Reaction with chlorine
The metals burn readily in chlorine to form white anhydrous chloride salts. Magnesium forms magnesium chloride and calcium forms calcium chloride.
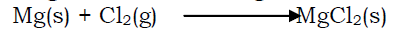

- Reaction with acids
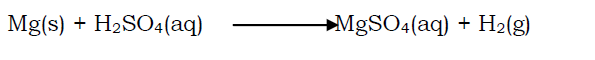
Calcium reacts vigorously with acids forming a salt and hydrogen gas. Magnesium reacts steadily with acids forming a salt and hydrogen gas. E.g. magnesium reacts with sulphuric acid forming magnesium sulphate and hydrogen gas.
Halogens
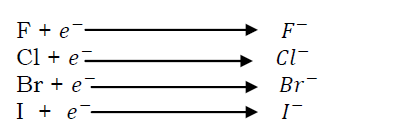
These are group (VII) elements; they are chlorine, bromine, iodine and fluorine. Halogens exist as diatomic molecules. This is because they lack one electron to completely fill their outer most energy level hence their atoms share electrons in order to completely fill the outer most energy level. These elements react by gaining one electron each forming negatively charged ions.
Physical properties
- They are colored (chlorine is greenish yellow gas; fluorine is pale yellow gas; bromine is a brown volatile liquid and iodine is a shinny black solid).
- Down the group, physical state changes from gas to liquid to solid. (fluorine and chlorine are gases at room temperature; bromine is a liquid and iodine is in solid form, the shinny black iodine solids sublime into a purple vapor on slight heating).
- Solubility in water decreases down the group. Fluorine, chlorine and bromine are soluble and iodine is slightly soluble in water.
- They have low melting and boiling points because the intermolecular forces of attraction are weak. The melting and boiling point increase down the group as the weak vanderwaal‘s forces of attraction increase with increase in atomic size.
Chemical properties
The order of reactivity decreases down the group.(i.e. fluorine is the most reactive and iodine in the least reactive). This is because these elements react by gaining electrons and down the group, the atomic radius increases there fore, an in coming electron is less readily attracted by the nucleus. There fore, the smaller the atom, the more readily and strongly does the nucleus attract an electron to the outer most shell and the more reactive that atom becomes. - Reaction with water
All the halogens dissolve in water forming a mixture of acids. Chlorine dissolves in water forming a mixture of hypochlorous acid(chloric (I) acid)(HOCl) and hydrochoric acid(HCl).

This solution turns blue litmus paper red then bleaches it. The bleaching action is due to the presence of hypochlorous acid(HOCl) that readily gives up its oxygen to the dye.
A solution of bromine in water is weakly acidic and weakly bleaching due to formation of weak hydrobromic acid (HBr) and bromic(I)acid(HOBr) respectively.
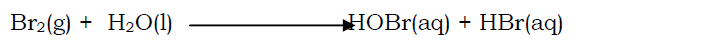
Chlorine is slightly soluble in water forming very weak acids. A solution of chlorine is too weak to bleach dyes, therefore, bleaching power of the elements decrease down the group.
- Reaction with alkalis
Halogen react with alkalis to form a mixture of salts and water. E.g. chlorine is absorbed by a solution of sodium hydroxide forming a pale yellow solution of a mixture of sodium chloride(NaCl) and sodium hypochlorite(NaOCl)
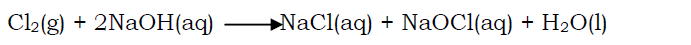
In hot concentrated solution of an alkali (e.g. NaOH), sodium hypochlorite is not formed but instead sodium hypochlorate is formed(NaOCl3).
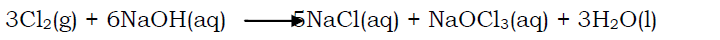
- Reaction with metals
Metals continue to burn in halogens forming salts. E.g. sodium continues to burn in a gas jar of chlorine to form dense white fumes which settle as white solids of sodium chloride. A similar reaction occurs with bromine and iodine.
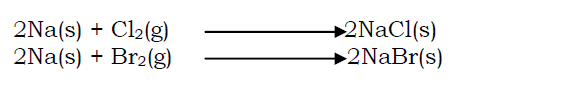
- Displacement reaction of halogens
When chlorine is bubbled through a colorless solution of potassium bromide, the solution gradually change to red-brown as bromine is displaced from its solution by chlorine.

If chlorine is bubbled through a colorless solution of potassium iodide,the solution turns dark brown as iodine is displaced from its solution.

Chlorine being the most reactive displaces all the halogens from their solutions.
