Back to: O level chemistry notes full Uganda syllabus
An alloy is a mixture of two or more metals. Alloys are formed by thoroughly mixing molten metals. It has been found that alloying produces metallic substance with more useful properties than the original pure metal it is made from. Examples of alloys include:
Differences between mixtures and compounds
Simple Criteria for purity
A pure substance is one which has distinct physical and chemical properties that are only unique it. These physical and chemical properties can be reproduced at ant time under the same conditions.
Physical properties such as taste, smell, and color of a substance cannot give accurate measurements of purity. For instance, sea water looks like pure water, impure
naphthalene has the same smell as pure naphthalene (smell of moth balls), pure copper wire feels like a wire made of copper alloy.
However, the following properties can be used to determine the degree of purity of substances. The values are constant for a pure substance.
Melting points of solids (is constant and the solid melts sharply )
Density of solids and liquids
Boiling point of liquids
Freezing point of liquids
Refractive index for liquids
The experimental values of the above properties are compared to the standard values and if they coincide, then the substance being investigated is pure.
N.B. The experiment for the investigation of purity must be carried out under the same conditions (of pressure and temperature) in which the standard values were obtained.
SOLUTIONS AND SUSPENSION
SOLUTIONS
A solution is a uniform mixture of two or more substances. Examples of solutions include: Air-a solution of gases; aqueous solution-a solution of any substance in water; alloy-a solution of metals.
Solutions are formed when solutes completely mix with solvents.
I.e. Solute + Solvent = Solution
A solute is a substance that dissolves in a solvent. Examples are salts and sugar.
A solvent is a substance that dissolves solutes. E.g. water.
A solute is said to be soluble in a given solvent if it can dissolve in the solvent and insoluble if it does not dissolve in that given solvent.
Depending on the amount of solute in the solvent, solutions can be classified as unsaturated, saturated and super saturated.
Unsaturated solution: This is a solution that can dissolve more solutes at a particular temperature.
Saturated solution: This is a solution that cannot dissolve any more solute at that temperature in the presence of undissolved solutes.
Super saturated solution: This is a solution that contains more solutes than it can hold at that temperature in the presence of undissolved solutes.
SUSPENSION
A suspension is a liquid containing small particles of solids which are spread throughout it and the solid particles settle on standing. Examples of suspensions include:
Paint – a suspension of colored substances in water or oil: Muddy water- a suspension of mud in water: suspension of chalk dust particles in water.
N.B. A suspension of a liquid in another liquid is called an emulsion and not a suspension.
Characteristics of a suspension
- Tinny solid particles are visibly seen spread throughout the liquid.
- On standing, the tinny solid particles settle at the bottom leaving a clear liquid. Centrifuging makes the solid particles to even settle faster.
- The tinny solid particles in a suspension can be separated from the liquid by filtration.
CRYSTALS
A crystal is a solid that consists of particles (atoms, molecules or ions) arranged in an orderly and repeatitive manner resulting into a definite shape. Crystals have regular shapes with flat sides and sharp edges. Examples of crystals include: sugar crystals, blue copper(II)sulphate crystals, common salt (sodium chloride) crystals, potassium nitrate crystals and potassium aluminium nitrate(Alum). Crystals are formed by the process of crystallization.
Crystallization is the process of evaporating a solution making it more saturated with the solutes such that the excess solutes are deposited as crystals. The solution is evaporated by either heating it or exposing it to sunlight.
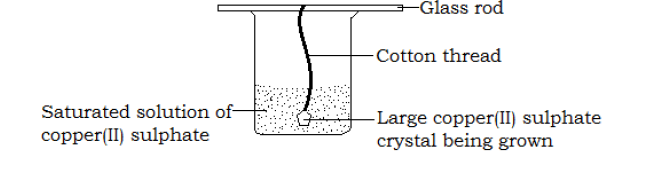
Growing of a large crystal
Large crystals can be grown from saturated solutions.
Growing of a large crystal of copper(II)sulphate.
Procedure
Drawing
- Pour a saturated solution of copper(II)sulphate in a beaker
- Dip a cotton thread into the solution and remove it. Allow the thread to dry, as small crystals form on it.
- Put back the thread containing small crystals into the saturated solution of copper(II)sulphate and allow the beaker to stand for some days in a warm place.
Observation
Large blue crystals of copper (II) sulphate will form on the cotton thread.
Water of crystallization
This is the definite amount of water with which some substances chemically combine when they form crystals (from their solutions in water). A compound that contains water of crystallization is referred to as a hydrated compound (hydrate). Examples of hydrated compounds include
Some crystals do not have water of crystallization e.g. sodium chloride (NaCl) and lead(II)nitrate (Pb(NO3)2).
Dry compounds that do not contain water of crystallization are called anhydrous compounds. E.g. anhydrous copper(II)sulphate (white powder); anhydrous calcium chloride (white powder). They normally exist in powdery forms and not as crystals.
Exchange of water takes place between some crystals and the atmosphere. Some substances absorb water from the atmosphere while some release water to the atmosphere. These substances are grouped in to three as:
- Hygroscopic substance
This is a substance that absorbs water from the atmosphere and remains physically unchanged. I.e. does not dissolve to form solution. Examples include: anhydrous copper(II)sulphate (CuSO4); calcium oxide (CaO); Copper(II)oxide (CuO);anhydrous calcium chloride (CaCl2) and concentrated sulphuric acid(H2SO4). Hygroscopic substances are used for drying gases.
Hygroscopy is the process of absorbing moisture/water from the atmosphere without the substance changing physically. - Deliquescent substance
This is a substance that absorbs water
from the atmosphere and dissolves in it to form a solution. E.g. sodium hydroxide pellets(NaOH); Potassium hydroxide pellets (KOH); and Copper(II)chloride (CuCl2).
Deliquescence is the process whereby a substance absorbs water from the atmosphere and forms a solution.
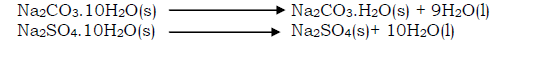
- Efflorescent substance
Is a substance that loses its water of crystallization to the atmosphere. The process in whereby a substance loses its water of crystallization to the atmosphere is called efflorescence. Examples of wfflorescent substances include; sodium carbonate decahydrate (Na2CO3.10H2O); Sodium suphate decahydrate (Na2SO4.10H2O).
When hydrated compounds are heated, they lose their crystalline shapes as the water of crystallization escapes, become powdery and as well their colours change.
For example when hydrated copper(II)crystals are heated, the blue crystals turn into white powder (anhydrous copper(II)sulphate) and a colorless liquid (water) condenses on the cooler parts of the test tube
Sample questions on Laboratory apparatus; Matter; Elements, Mixtures and compounds
Bunsen burner and flames
- A Bunsen burner is one of the apparatus used for heating in the laboratory.
a) Define the term laboratory
b) Make a labeled drawing of a Bunsen burner and give the functions of all labeled parts.
c) How are you able to light a Bunsen burner? - Define a flame? Mention the two common types of flames and conditions under which each is produced. Make labeled drawings of the two types of flames and explain what happens in each of the labeled zones. Give four differences and two similarities between the two types of flames. What is strike back?
Matter - What is matter? Mention the different states of matter and give the characteristics of each state.
- State kinetic theory of matter? Use kinetic theory of matter to explain why a solid object changes into a liquid and finally a gas when heated. Uses a schematic diagram to illustrate the different processes involved in the transition of matter from one state to another. Define the processes mentioned in the schematic diagram.
- Define Brownian motion and diffusion. Describe experiments to demonstrate Brownian motion and diffusion in liquids and gases. Outline four factors that affect the rate of diffusion of gases. Describe an experiment to show that ammonia and hydrogen chloride gases diffuse at different rates.
- Define the terms melting, freezing and boiling points. Describe experiments to show how you can determine the melting point of naphthalene and boiling point of ethanol. Sketch a temperature-time graph for cooling liquid naphthalene. Identify the melting point and label it T on the graph axis.
- Define the terms physical and chemical changes and state at least two examples of each. Mention the characteristics of physical and chemical changes and clearly give the differences between them.
- Describe the observations you would make when each of the following substances are heated: candle wax; magnesium, iodine; potassium permanganate; lead(II) nitrate; hydrated copper(II) sulphate; sulphur and zinc oxide.
Elements, mixtures and compounds - Define the terms element, mixture and compounds giving three examples of each (where applicable write the symbol or formula). Give the names and formulae of five compounds stating clearly the elements that they are composed of. What are the differences between a mixture and a compound?
- On what property is each of the following methods of separation of mixtures based: fractional distillation; fractional crystallization; sublimation; chromatography and magnetic separation?
- Describe how the following mixtures can be separated: oil and water; salt from water; sugar from sand; sand and water; ethanol and water; diesel and kerosene; a mixture of different colours; iodine and common salt; potassium chloride and potassium nitrate; iron and sulphur.
- What an alloy, mention three examples is of alloys any their uses. Brass, bronze, duralumin, solder and stainless steel are alloys. Mention the components in each alloy.
Solutions, suspension and crystals - Define the following terms as applied to chemistry: solution, suspension, solute, solvent, saturated solution, unsaturated solution, super saturated solution and crystallization.
- What is a crystal? Explain with a the aid of a drawing how a large crystal of copper(II) sulphate can be grown.
- Some compounds are said to have water of crystallization. What is water of crystallization? Mention three examples of hydrated compounds and write their formulae.
- Define the following terms: hygroscopy, deliquescence and efflorescence. What are hygroscopic and efflorescent substances (illustrate with at least two examples of each)