Back to: O level chemistry notes full Uganda syllabus
a) CHEMICAL (PERMANENT) CHANGE
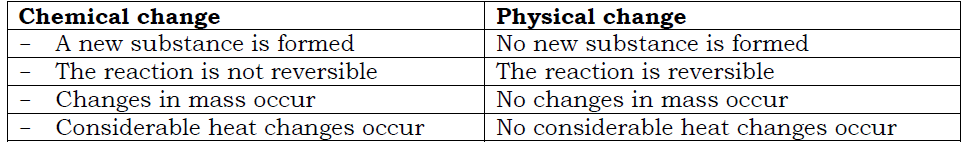
This is a change in which a new substance is formed and the change is irreversible i.e the substance formed cannot be turned back to its original form.
Characteristics of chemical changes
- Great heat changes occur i.e. heat is either absorbed or released.
- Changes in mass occur i.e. there is increase or decrease in the mass of the product formed.
- New substances are formed during chemical changes.
- The reaction is not reversible (irreversible).
Examples of chemical changes include: burning of charcoal; rusting of iron; burning of substances in air e.g. magnesium; addition of water to calcium oxide; explosion of natural gas or hydrogen with air.
b) PHYSICAL (NON PERMANENT) CHANGE
This is a change in which no new substance is formed and the change is reversible.i.e the substance formed can be converted back to its original form.
Characteristics of physical changes
- The reaction is not accompanied by great heat changes(except latent heat changes accompanying the reaction)
- No new substance is formed
- No changes in mass occurs i.e. the mass of the product is the same as that of the reactant.
- The reaction is reversible.
Examples of physical changes include: melting of ice; vaporization of a liquid; magnetization of iron; sublimation of iodine.
Differences between physical and chemical changes
Effects of heat on some substances
a) Magnesium
When magnesium is burnt, it burns with a brilliant white flame producing white fumes and white ash.
b) Iodine
When the grey shinny solids of iodine are heated in a test tube, it forms a purple vapor which settles as grey shinny solids at the cooler parts of the test tube.
c) Candle wax
When candle wax is heated in a test tube, it forms a colorless liquid which turns back into a solid on cooling.
d) Sulphur
When sulphur is heated gently in a test tube, it melts into a brown liquid and on cooling, it turn to yellow solids. However, when heated strongly, itburns with a blue flame producing a colorless gas with a choking smell.
e) Potassium permanganate
When potassium permanganate is heated in a boiling tube, it decomposes with a cracking sound producing black fumes and black powder.
f) Copper (II) sulphate crystals (Hydrated)
When the blue copper (II) sulphate crystals are heated in a test tube, a colorless vapor condenses at the cooler part of the test tube forming a colorless liquid and the blue crystals turn to white powder. However when water is added to the white powder, blue crystals of copper (II) sulphate are formed back.
g) Zinc oxide
When white powder of zinc oxide is heated in a boiling tube, it turns to yellow and on cooling in turns back to white.
h) Nicrome wire
On heating nicrome wire, it glows white hot and on cooling turns back into its original state.
i) Lead (II)nitrate
When the lead (II) nitrate is heated in a test tube, it burns with a cracking sound and melts into brown liquid producing fumes of brown gas with an irritating smell and finally yellow residue is left in the test tube.
CLASSIFICATION OF SUBSTANCES
In chemistry, substances are classified as elements, compounds and mixtures.
ELEMENTS
An element is a pure substance that cannot be split or divided into two or more simpler forms by any known chemical means. An element is made up of atoms.
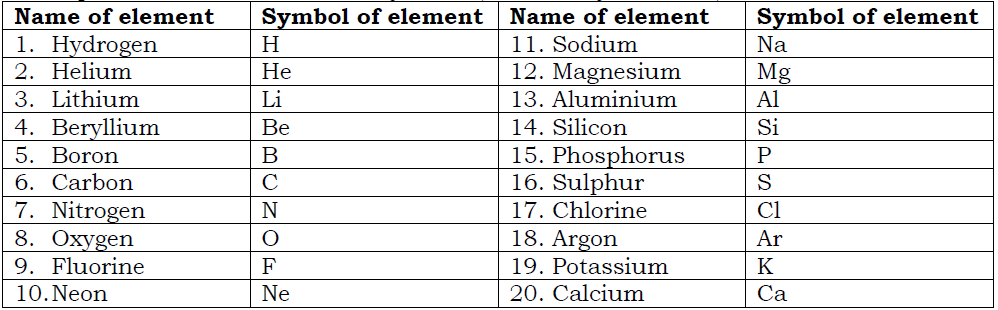
Examples of elements with their symbols (first twenty elements).
Other examples of elements and their symbols include: Copper (Cu);Zinc (Zn);Iron (Fe);Mercury (Hg);Lead (Pb);Gold (Au);Bromine (Br)Cobalt (Co);Manganese (Mn).
Naming of elements
In naming elements, the 1st letter or the 1st two letters are used as symbols for the elements.
Examples
However, there are elements whose symbols are different from the 1st letters of their names. This is because; the symbols used are of old latin names. Examples of these elements include the following.
COMPOUNDS
A compound is a substance made up of two of more elements chemically combined together. Examples of compounds include the following.

During the formation of compounds (chemicals combination of elements), atoms of elements combine in definite proportions and energy in the form of heat, light or electrical form is either released or absorbed.
MIXTURES
A mixture is a substance made up of two or more elements or compounds physically combined together.
Classes of mixture
- Solid-solid mixture e.g. copper and tin, salt and sugar, sand and salt.
- Solid-liquid mixture e.g. sand and water, chalk and water.
- Liquid-liquid mixture e.g. ethanol and water, oil and water.
- Liquid-gas mixture e.g. water and air.
- Gas-gas mixture e.g. carbondioxide and oxygen, nitrogen and oxygen.
SEPARATION OF MIXTURES
Mixtures can be separated by a number of physical methods depending on the properties of the components such as solubility, boiling point and magnetic properties. Some common methods of separating mixtures include: decantation, filtration, centrifuge method, sublimation method, use of a separating funnel, evaporation, simple distillation, fractional distillation, separation by a magnet and chromatography.
a) Separation of insoluble solids from liquids e.g. sand from water. The methods used are: Decantation, centrifuging and filtration.
- Decantation
The mixture of the solid (sand) and liquid (water) is poured in a beaker and allowed to stand for a while. The solids (sand) being denser will settle at the bottom of the beaker while the liquid (water) remains on top as it is less denser than sand. The liquid (water) is carefully poured into another beaker leaving sand in the beaker that had the mixture.
Drawing to illustrate
Note:
Sometimes, the solid particles may be very tinny and may take time to settle at the bottom therefore separation by decantation may be very difficult. For such a mixture, the solids can be made to settle faster at the bottom of the container by using a centrifuge. A centrifuge is a machine that speeds up the process of sedimentation (settling of solids).
During centrifuging, test tubes containing the mixture are spun at a very high speed and the solids are thrown at the bottom of the test tubes. Separation by decantation can then be possible.
Drawing to illustrate
- Filtration
During filtration, the mixture of the solid (sand) and liquid(water) is poured through a filter funnel containing a filter paper. The solid particles remain trapped on the filter paper and is called residue while the liquid passes through the filter paper and is collected in another beaker as the filtrate.
Drawing to illustrate
b) Separation of a solute from a solvent e.g. sugar (salt) from water. Methods used include: evaporation and simple distillation.
A solute (e.g. salt) is a substance that dissolves in a solvent (e.g. water) and a solvent is a substance that dissolves a solute. A mixture of the solute and solvent forms a solution (e.g. salt solution).
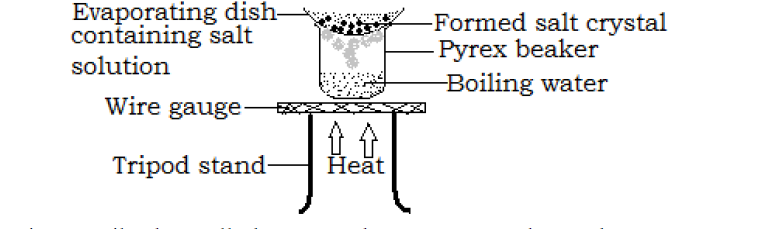
- Evaporation method
In this method, the solution is put in an evaporating dish and heated gently over a steam bath as shown below.
Drawing to illustrate
Continue heating until when all the water has evaporated. As the water evaporates, crystals of the salt or sugar are formed in the evaporating dish (crystallization occurs).
- Simple distillation
Distillation is the process of heating a liquid to form vapor and condensing the vapor to form a more pure liquid. This method can be used to separate solutes from solvents and for separating liquid mixtures whose boiling points are wide apart(i.e. separating liquids with a big difference in boiling point.) such as ink and water.
Separating salt from water by simple distillation
The salt solution is placed in a distillation flask and the apparatus arranged as shown below.
When the mixture is heated, the solvent (water) being more volatile will escape and its vapor condensed by the Liebig condenser forming the distillate. The solute (salt) will remain at the bottom of the distillation flask as crystals.
c) Separation of miscible liquids
Miscible liquids are liquids that can be mixed together in all proportion forming a uniform layer. The uniform mixtures of two or more liquids form a homogenous mixture. Examples of uniform mixtures are: ethanol and water, ethanol and propanol, water and ink e.t.c. the homogenous mixtures (uniform mixtures) can be separated by simple distillation (when the differences in boiling points of the liquids are wide) and fractional distillation (when the boiling point difference of the liquids is narrow).
Separation of a mixture of ink and water by simple distillation
Procedure
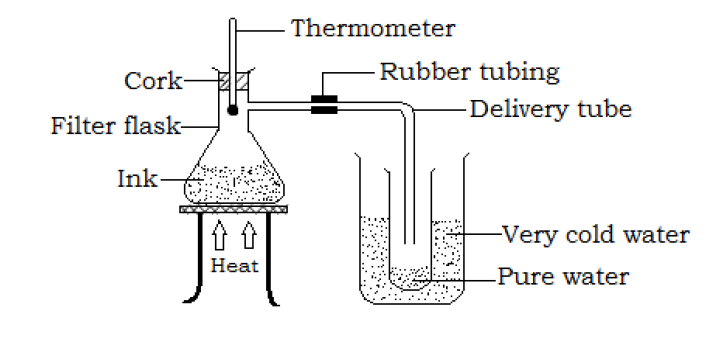
- Pour a mixture of ink and water into a filter flask fitted with a delivery tube to its side arm and insert a rubber bung carrying a thermometer to its top as shown below.
The delivery tube at the side arm is connected to an empty test tube placed in cold water (the cold water is to condense the gas vapor)
- Heat the mixture (ink solution) carefully and note the temperature at which the solution is boiling. As heating in continued, the water vapor escapes from the solution since its boiling point is lower and condenses in the test tube. The temperature remains steady and this will be recorded as the boiling point of water.
- Slightly above the steady temperature, heating is stopped as the water has been separated from the ink. The water is collected as a distillate and the ink remains in the filter flask.
N.B. Do not continue to heat until all the liquid has been transferred from the flask to the test tube. Stop heating shortly when the temperature remains steady.
- Fractional distillation
This is a process of separating two liquids by using their differences in boiling point. Liquids to be separated by this method must have different boiling points. This method can be used in separating ethanol and water; separating a mixture of gases; petroleum refining and brewing industries.
Separating a mixture of ethanol and water by fractional distillation
Set up of apparatus
Procedure
- Place the mixture of ethanol and and water in the distillation flask and arranged the apparatus as shown above.
Heat the mixture until when it begins to boil. Ethanol having a lower boiling point (78˚C) boils first leaving the water in the distillation flask. The temperature remains constant for a while as the ethanol vapor rises up the fractionating column and is condensed by the Liebig condenser forming a colorless distillate (ethanol)
N.B. 1. Water vapor may also try to escape but it cannot go up the fractionating column as it condenses and falls back into the distillation flask.
- The beads in the fractionating column increase the surface area for condensation of the rising vapor.
- When the temperature rises slightly above the boiling point of ethanol (78˚C). Remove the distillate and this will be pure ethanol. The water remains in the distillation flask and may be collected as the second distillate if heating is continued up to 100˚C.
N.B. Anti bumping stones (broken porcelain) is added to the mixture of ethanol and water to prevent violent boiling of the mixture
Applications of fractional distillation - It‘s used in the brewing industry to produce quality drinks such as beer, whisky and distilled waragi.
- It‘s used in the oil refining industry to separate the different components of crude oil like kerosene, diesel and petrol.
- It‘s used in large scale manufacture of oxygen. Liquid air can be fractionally distilled and its components separated.
d) Separation of immiscible mixtures/liquids(Heterogeneous mixture)
Heterogeneous mixture is a non uniform mixture of two or substances. Each of the substances may form separate layers.
Immiscible liquids are liquids which do not mix in any proportion with each other. They therefore form separate layers e.g. a mixture of oil and water, paraffin and water. Such a mixture can be separated by use of a separating funnel.
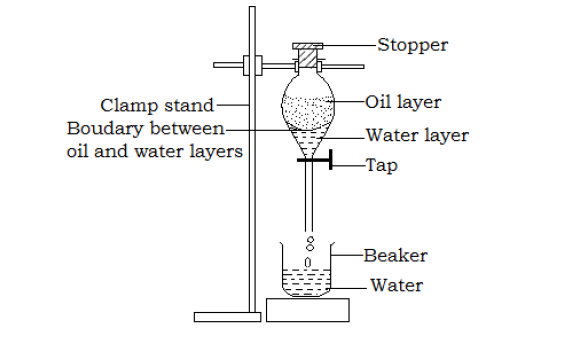
- Use of a separating funnel
A separating funnel is used to separate liquids that do not mix like oil and water.
Separation of oil and water using a separating funnel
Set up of apparatus
Procedure
- A mixture of oil and water is poured into the separating funnel when the tap is closed.
- The mixture is allowed to settle and oil being less dense than water will remain at the top and water at the bottom.
- Carefully open the tap and run the water in an empty beaker placed below the separating funnel. As soon as the water has run into the beaker, close the tap and the oil remains in the separating funnel.
e) Separation of volatile solids from non volatile solids
Solids that can sublime (volatile solids) can be separated from solids that cannot sublime by sublimation method.
- Sublimation method
Sublimation is the direct change of state of a substance from solid to gas and from a gas to solid without going through the liquid state. Sublimation method can be used to separate:
- Volatile solids from their solution e.g. iodine solids from its solution in water.
- Volatile solids mixed with non volatile solids e.g. iodine from common salt.
Examples of substances that can sublime are: iodine, ammonium chloride, anhydrous iron(III) chloride, anhydrous aluminium chloride and benzoic acid.
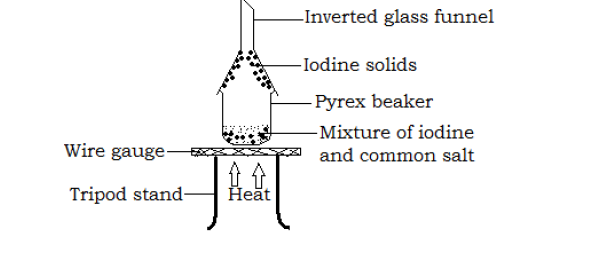
Separation of iodine from common salt
Set up of apparatus
Procedure
- A mixture of equal amounts of iodine and common salt (sodium chloride) is placed in a beaker with a glass filter funnel inverted over its top as shown above.
- The beaker is then heated gently.
Observation
Iodine solids sublime forming a purple vapor that settles at the inside of the filter funnel forming grey solids of iodine. White common salt is left in the Pyrex beaker.
f) Separation of magnetic substances from non magnetic substances
Magnetic substances are attracted to a magnet while non magnetic substances are not, there fore they can be separated by magnetic method.
- Magnetic separation
This method can be used to separate feromagnetic substances like iron fillings from non magnetic substances like sulphur and glass.
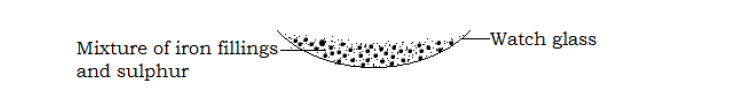
Separation of iron fillings from sulphur
Procedure
- Place a mixture of iron fillings and sulphur on a watch glass. As shown below.
Pass a bar of magnet over the mixture.
Observation
- The iron fillings were attracted to the bar magnet and clung to it while the sulphur was left in the watch glass because they are non magnetic.
Separation of different solutes e.g. salts dissolved in a solvent
Two of more solutes (salts) can dissolve in a particular solvent and can be separated from each other on the basis of their differences in solubility in the same solvent. E.g. a mixture of potassium chlorate and potassium chloride can be separated from solution. The method of separation used is fractional crystallization. Another method that can be used to separate a mixture of two solutes in a solution is solvent extraction.
- Fractional crystallization
This is a method of separating two or more soluble solutes by crystallization using their differences in solubility in the same solvent.
Crystallization is the process of forming crystals by evaporating a saturated solution. I.e. the solution is made more and more saturated by evaporating it so that the excess solutes are deposited as crystals.
In fractional crystallization, a mixture of two solutes (e.g. salts) with different solubilities is dissolved in a minimum amount of hot solvent to make a saturated solution. The solution is then allowed to cool and as it cools, the less soluble salt crystallizes out (forms crystals). It is then filtered and the more soluble salt remains in the filtrate. The crystals are again dissolved in a minimum amount of hot solvent and cooled again to form crystals. The process is repeated until pure crystals of less soluble solutes are separated from the mixture.
In other words, as the hot solution cools, the solutes with different solubilities form crystals at different temperatures. - Solvent extraction
This method can be solutes from a solution in which more than one solute is dissolved. E.g. a solution containing iodine and sodium chloride dissolved in water. Solvent extraction works on two principles:
- One solute in the solution must be more soluble in the extracting solvent.
- The extracting solvent must not be miscible with the solvent in which the solutes are dissolved.
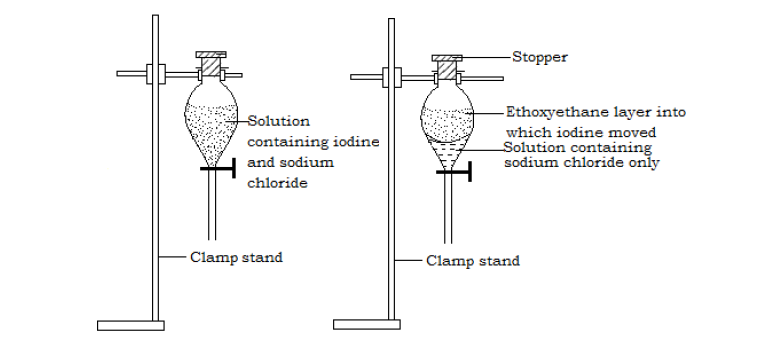
Separation of iodine from a solution containing iodine and sodium chloride using ethoxyethane as the extracting solvent
Procedure - A mixture of iodine and sodium chloride solution is poured into a separating funnel.
- Ethoxyethane solvent is added on to the mixture and the stopper placed on top of the funnel. The funnel is then shaken and the mixture left to settle.
Results
The ethoxyethane layer is less dense and settles on top. Iodine being more soluble in the ethoxyethane solvent than in water passes into the ethoxyethane leaving sodium chloride in the water solvent.
h) Separation of colored substances (dyes)
A mixture of colored substance is separated by chromatography.
- Chromatography
This is the process of separating a mixture of colored substances with different rates of movement in a solvent over an adsorbent medium. The adsorbent medium should be stationary and porous. This method can be used in separating different components in ink, pigments in leaves or grasses. There are different types of chromatography but the most common one is paper chromatography in which the stationary medium is a porous paper e.g. filters paper.
The dyes (colored substances) separated by this method should be soluble in the solvent and should be absorbed to some extent by the stationary medium (adsorbent medium). The pigment (dye) that is more soluble in the solvent is less absorbed (has les tendency to stick to the paper) and there fore moves faster. The dye that is less soluble has a great tendency of being absorbed and travels a very short distance.
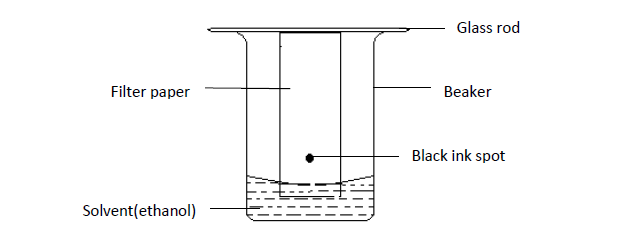
Separation of the components in black ink
Procedure
- Mark a spot on a strip of filter paper using black ink.
- Suspend the filter paper with its tip in a beaker containing ethanol as shown below.
- After some times, the filter paper was removed from the solvent (ethanol) and allowed to dry.
Results
The chromatogram of the different components of black ink is as shown below.
The chromatogram is the paper that shows the different components (colors) of the mixture separated from each other.
The solvent (ethanol) moves up the filter paper and dissolves the colored components in the black ink, then moves with them. The colored components moved upwards at different rates depending on their solubility in the ethanol. The most soluble component (yellow) moved the greatest distance as it was less absorbed by the filter paper and vice versa.
N.B. If two of more mixtures are separated using the same chromatographic paper, the components which are common in both mixtures travel the same distance.
Example to illustrate
Consider the chromatogram of mixtures A,B,C and D(which are all different mixtures) shown below.
Alternative procedure for separating components in black ink
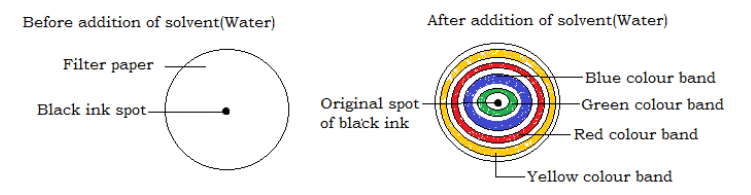
- Place a filter paper on a flat surface and a drop of black ink to the centre of the filter paper; allow the ink on the paper to dry.
- Add a drop of water (solvent) to the dried ink spot and allow it to dry before you add another drop of water i.e. for every drop of water, allow it to dry before you add another drop.
- Repeat the addition of water until the band of colored substances almost reaches the edge of the filter paper.
Observation
As the solvent (water) moved across the filter paper, it carried a long with it the different dyes in black in and separated them. The dyes separated because they have different solubilites in water and are absorbed to different degrees by the porous filter paper. Yellow was the most soluble in water and least absorbed by the filter paper there fore travelled the longest distance. Green was least soluble in water and most absorbed by the filter paper there fore travelled the shortest distance.
The ratio of distance moved by the solute (dye) to the distance moved by the solvent in chromatography is called, the retention factor (Rf).
Exercise
- Sharron wants to separate the liquid from the dye in black ink. She uses this apparatus to distill the liquid.
Copy the diagram and add to it a thermometer in a correct position to measure the boiling point of the liquid which distilled over first.
a) Why was ice put in the beaker around the test tube.
b) The black dye in ink was thought to have a boiling point of either 54˚C, 93˚C or 150˚C
Sharron collected the first liquid in the tes tube and found that its boiling point was 100˚C. The liquid was colorless. State with a reason which of the three temperatures is the boiling point of the dye in black ink.
c) Sharron tested another black ink to see if more than one color was present in it. She made chromatograms by placing drops of the liquid to be tested on a pencil line drawn on a filter paper. She stood the paper vertically in a suitable solvent for some hours.
Figure 1 shows the chromatogram of some known dryes.
Figure 2 shows the chromatogram of the black ink.
i) Which dyes are present in the black ink
ii) Suggest why the starting line is drawn in pencil