Back to: O level chemistry notes full Uganda syllabus
The study of chemical reactions and factors that affect the rates of chemical reactions is known as chemical kinetics.
The rate of a chemical reaction is the speed at which products are formed or reactants are used up in a chemical reaction.
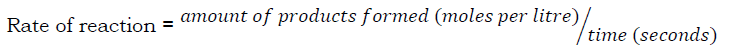
Most reactions occur in solutions and the amount is usually measured in moles/litre and time is measured in seconds, therefore, the unit of rate of reaction is mol/l/s.
Rates of reactions indicate how fast reactions are occurring, some reactions occur very rapidly e.g. explosions and precipitation while others occur very slowly e.g. rusting and fermentation. Some reactions proceed at a moderate rate e.g. reaction between hydrochloric acid and zinc metals.
Rate curves
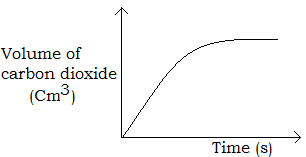
The change in concentration (amount) of reactants of products with time is often plotted as the rate curve (figure a).

The rate of reaction at any time, t can be found from the rate curve by drawing a tangent at that time as shown in figure (b). The gradient of the tangent is obtained as the rate of reaction at that time.
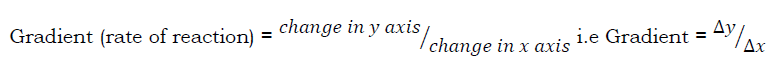
Determination of rate of reaction by
a) Change in gas volume
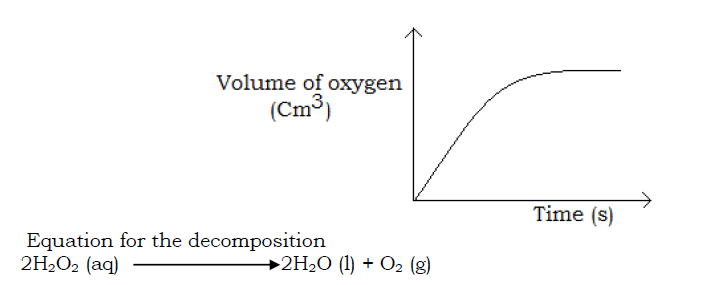
In reactions in which gases are formed, the volume of the gas can be recorded at various times. The rate curve is drawn and used to determine the rate of reaction. Examples are the decomposition of hydrogen peroxide and reaction of an acid and a metal.
i) Decomposition of hydrogen peroxide

Procedure
- A known volume of hydrogen peroxide is put in a flask as shown above and manganese (IV) oxide added to it.
- A rubber bung connected to a gas syringe is immediately inserted to close the flask. The stop clock is started the same time the flask is closed.
- The volume of oxygen gas collected in the syringe is read and recorded after a fixed time interval until when the reaction stops.
- The volume of gas evolved is then plotted against time. A rate curve as below is obtained.
ii) Measuring volume of carbon dioxide produced when calcium carbonate reacts with hydrochloric acid
Procedure
- Place a known volume of dilute hydrochloric acid in a flask as shown below and add a known mass of calcium carbonate.
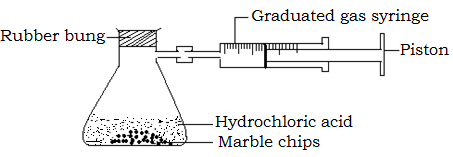
- A rubber bung connected to a gas syringe is immediately inserted to close the flask. The stop clock is started the same time the flask is closed.
- The volume of carbon dioxide gas collected in the syringe is read and recorded after a fixed time interval until when the reaction stops.
- The volume of carbon dioxide gas evolved is then plotted against time. A rate curve as below is obtained.

formed dissolves in the reaction mixture (solution), it is necessary to first saturate the solution with carbon dioxide so that all the carbon dioxide produced from the known mass is wholly measured.
b) Change in mass
Measuring the mass of the reaction mixture as carbon dioxide is evolved from reaction of calcium carbonate and hydrochloric acid
Set up

Procedure
- A flask containing a known volume of hydrochloric acid is weighed using a direct reading balance.
- A known mass of marble chips is added carefully and a rubber bung carrying glass tubing with cotton wool is immediately inserted to close the flask. A stop clock is started at the same time.
- The mass of the flask and its content is recorded at a regular interval of time.
- The results are then plotted on a graph.

The gradient is steep at the start of the reaction and gradually reduces until when it finally becomes zero (where it levels off) – at the end of the reaction. This implies that the rate of reaction is highest at the start and gradually decreases until when it becomes zero at the end of the reaction. This is because, at the start of the reaction, the concentration (amount) of reactants is highest but keeps on decreasing until when it becomes zero at the end of the reaction when all of it (reactants) have been consumed or used up.
The collision theory and rate of reaction
The collision theory states that before two or more substances can react, they must first collide. During a chemical reaction, molecules of reactants tend to approach one another, collide then chemical reactions take on. Therefore, the rate of chemical reaction depends on how close together the molecules of reactants are and how fast they are moving. Consequently the frequency of collision of molecules of reactants and rate of reaction is affected.
Factors affecting rates of reactions
A number of factors influence the rate of chemical reactions and these include: temperature, surface area (size of particles), concentration of reactants, light, catalyst, and pressure (especially for gaseous reactions).
- Temperature
The rate of chemical reactions increase with increase in temperature. For every 10K rise in temperature, the rate of reaction is approximately doubled.
Explanation
When temperature increases, the molecules/ions of reactants gain more kinetic energy and tend to move faster. Their frequency of collision consequently increases which results into increased rate of reaction. Therefore, the higher the temperature, the higher the rate of reactions.
Example
Hydrogen peroxide decomposes at room temperature in the presence of manganese (IV) oxide to produce oxygen. The rate at which oxygen gas was produced at different temperatures was determined by measuring the volume of oxygen gas evolved at different temperatures and the graph below was obtained.
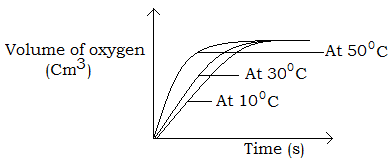
The graphs all end at the same level as the same amount of reactants were used.
- Surface area (size of the particles)
An increase in the surface area of the particles of solid reactants increases the rate of reaction if other factors are kept constant. This is because; increase in surface area increases the frequency of collision. Consequently, solids react more faster when in powdery form than when in large lumps.
Example
Calcium carbonate reacts more rapidly with hydrochloric acid when in powdery form than when in form of marble chips. This is because the powder form has a large surface area exposed and more readily collide with the acid.
Graphical illustration
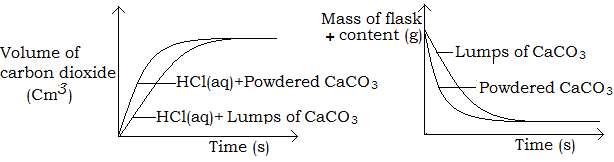
- Concentration of reactants
Concentration refers to how close together the solute particles are in a given solution.
Increasing the concentration of reactants increase the rate of a chemical reaction. The higher the concentration, the closer are the solute particles the higher the frequency of collision of the solute particles and this results into an increase in the rate of reaction.
Examples
i) Reaction of zinc and dilute hydrochloric acid
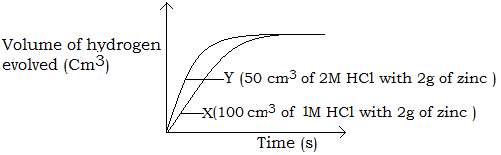
When 100cm3 of 1M HCl was reacted with 2g of Zinc and the volume of hydrogen gas evolved measured at regular time interval is plotted against time, curve X is obtained. Curve Y is obtained when 50 cm3 of 2M HCl reacted with 2 g of zinc.
The gradient of curve Y is steeper than the gradient of curve X, therefore, the rate of reaction in Y is higher than in X. This is because in Y, a more concentrated acid was used than in X and the number of hydrogen ions per unit volume is higher in Y making them to collide more frequently and react more often than in X.
The gradient of Y is approximately twice that of X, because the acid used in Y is twice more concentrated as that used in X.
The curve for Y levels off first because the hydrochloric acid used in Y being more concentrated makes the reaction to reach completion much earlier. The maximum volume of gas (hydrogen) produced in X and Y is the same since the number of moles of hydrogen ions present in both acids are the same.
ii) Reaction of hydrochloric acid and sodium thiosulphate
Reaction of dilute hydrochloric acid and sodium thiosulphate produces precipitates of sulphur. The intensity of the precipitate can be studied by placing the beaker with the two reactants over a white tile with a mark on it as shown below.
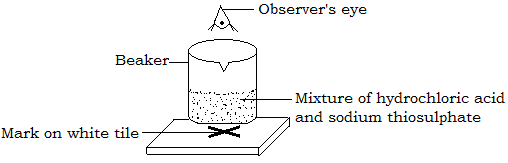
The precipitates will eventually cover the mark. The time taken for the mark to disappear from view is recorded. The time for disappearance of the mark for different concentrations of the acid and thiosulphate are recorded.
If the concentration of the dilute hydrochloric acid or thiosulphate is increased, the time for disappearance of the mark decreases hence the rate is faster when the solutions are made more concentrated.
Trial question
Procedure
- Mark a cross with blue or black ink on a piece of paper.
- Place 50cm3 of sodium thiosulphate solution in a beaker.
- Add 5.0cm3 of 2M hydrochloric acid provided to the sodium thiosulphate solution and start the stop clock immediately.
- Shake the beaker gently for the solution to mix well and then place it on the paper over the cross.
- Watch the cross through the solution. i.e. from above the beaker.
- Stop the clock as soon as the cross disappears.
- Record the time taken for the cross to disappear.
- Pour away the mixture and rinse the beaker thoroughly.
- Place 40cm3 of thiosulphate solution and then add 10cm3 of water into the solution.
- Added 5 cm3 of the acid as before and then follow the procedure given above. Record the time taken for the cross to disappear.
- Repeat the above procedure for the rest of the mixture as shown in the table below.
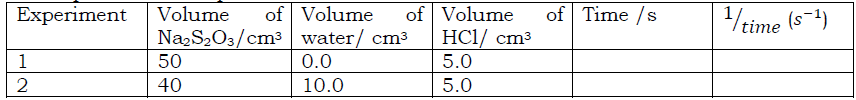

a) Write an ionic equation for the reaction.
b) Why does the cross disappear in the experiment?
c) Plot a graph of volume of thiosulphate against time (describe and explain the shape of the graph)
d) Plot a graph of 1⁄time against volume of thiosulphate solution (calculate the gradient/ slope of your graph and state its unit)
- Pressure
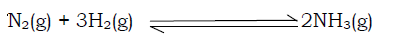
This affects gaseous reactions since gases unlike solids are compressible. Increasing pressure on gases brings the reactant particles close to each other increasing the frequency of collision and hence the rate of reaction. Pressure can be increased by decreasing the volume of the container. For example in the Haber process, a large yield of ammonia is obtained from high pressure as per the following reaction.
- Light
Light is another form of energy that speeds up rates of chemical reactions. Reactions whose rates are affected by light are said to be photosensitive. Examples of such reactions include:
i) Reaction of hydrogen and chlorine which occurs explosively in the presence of light.

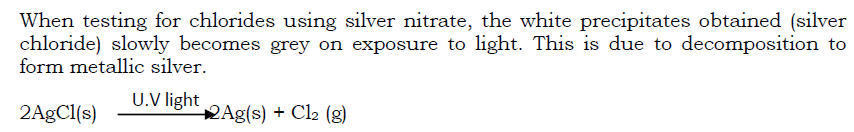
6. Catalyst
A catalyst is a substance that alters the rate of a chemical reaction but remains chemically unchanged at the end of the reaction. A catalyst may change physically during the course of a reaction but not chemically.
NB. To prove that catalysts do not change chemically, weigh the mass of the catalyst before and after a reaction- the mass does not change.
A catalyst (positive catalyst) lowers the activation energy required to initiate a chemical reaction and consequently increases the rate of the reaction.
Illustration
The rate of decomposition of hydrogen peroxide is low without a catalyst and increases when a catalyst (manganese (IV) oxide) is added.

At the beginning, the curves are steep but get less steep as time goes by until the end of the reaction where they level off. The rate of reaction is highest at the beginning but decreases steadily throughout the reaction until when the reaction stops. This is because the concentration of hydrogen peroxide decreases as the reaction goes on.
Curve for the reaction that had a catalyst has a steeper gradient and levels off before the curve for reaction without a catalyst. This is because; a catalyst ensures a more effective collision per second and therefore increasing the rate of reaction.
Some catalysts lower the rate of chemical reactions and are known as negative catalysts.
Chemical reactions and equilibrium/’balance’
Chemical reactions can further be sub divided into classes of reactions each of which has its own characteristics and a few of which are: combination, displacement, decomposition and double decomposition.
i) Combination
This takes place when two or more substances combine to form a single substance. E.g when iron is heated with sulphur to form iron (II)sulphide.

When lead (IV) oxide is lowered into a gas jar of sulphur dioxide, they combine and form lead (II)sulphate.

ii) Decomposition
This occurs when a compound spits up into simpler substances. This change usually takes place without the presence of a second substance and very often the action of heat is sufficient to cause the reaction to take place. E.g. When calcium carbonate is heated in a crucible to bright red, it decomposes to form calcium oxide and carbon dioxide.

iii) Displacement
This occurs when one element (or group) takes the place of another element (or group) in a compound. E.g. if zinc is placed in copper (II) sulphate solution, copper is displaced by zinc and zinc sulphate is left.

When chlorine is bubbled through a solution of potassium bromide, the chlorine displaces bromine and red bubbles of bromine are formed. A solution of potassium bromide is left.

iv) Double decomposition
In this reaction, two compounds take part, both are decomposed and two new substances are formed by exchange of radicals. In most cases both reactants are soluble in water and only one of the products is soluble. The insoluble product is precipitated out in form of solids (it is this product that is normally wanted).
When hydrogen sulphide gas is bubbled through a solution of copper (II)sulphate, copper (II)sulphide and sulphuric acid are formed.

Less frequently is the wanted product of double decomposition more volatile and it is driven of as a gas or by heating as a vapour of a more volatile liquid.

Reversible reactions
Most reactions proceed in only the forward direction until when one of the reactants or all the reactants are used up then the reaction stops. Such a reaction is known as an irreversible reaction.
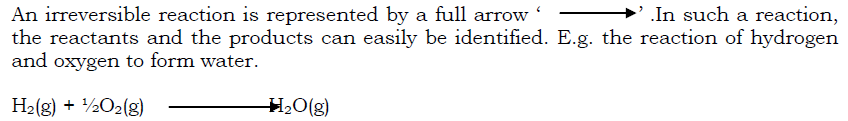
However, there are some reactions in which the direction of chemical change can be reversed by changing the conditions under which the reaction is taking place. The reaction can proceed in both forward and backward directions depending on the conditions to which the reaction is subjected i.e. the products can react to form back the substances that were initially reacting. Such a reaction is known as a reversible reaction. E.g when hydrated copper (II) sulphate is heated, the blue colour of the crystals changes to white due to formation of anhydrous copper (II) sulphate.
Chemical balance (chemical equilibrium)
In reversible reactions, both forward and backward reactions can take place at the same time and the reaction might come to some kind of a ‗balance‘ in which the products and reactants are present at the same time.
Suppose n molecules of substance A reacts with m molecules of substance B to form x molecules of C and y molecules of D as shown below,
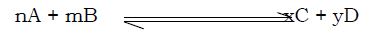
As soon as a little of C and D are formed, a reverse reaction will begin. At first, the forward reaction will predominate, but, as C and D accumulate, the reverse reaction will build up until when a ‗balance, (equilibrium position) is reached with both the forward and backward reactions proceeding at the same rate. The composition of the mixture will appear to be constant though it is the net result of the two opposing reactions.
Factors affecting chemical equilibrium (balance)
The factors affecting equilibrium position were investigated by Louis Henri Le Chatelier and he summarized it in a statement known as Le Chatelier‘s principle. The principle states that
“if an external condition is applied to a reaction in equilibrium, the equilibrium will shift to a direction which tends to cancel the effect of the applied condition”
These factors that affect equilibrium position are temperature, pressure (for gases), concentration and catalyst.
i) Temperature
The effect of temperature depends on whether the reaction is exothermic or endothermic.
If a reaction is exothermic (gives out heat) and heat is applied to the system, the reaction shifts in a direction that tends to lower the temperature i.e. the reverse reaction occurs. Consider the reaction below in the Haber process of manufacturing ammonia.

Since the reaction is exothermic, when temperature is raised, the reverse reaction which consumes heat occurs i.e. more of nitrogen and hydrogen will be produced from ammonia. The backward reaction is thus favoured.
If the reaction is endothermic (absorbs heat) and the temperature of the system is raised, the reaction proceeds in such a way that the heat applied is used up. The reaction proceeds in a forward direction.
ii) Pressure
A change in pressure affects mainly reactions which involve gases. The effect of pressure depends on whether there is an increase or decrease in number of molecules. Reactions that do not proceed with change in the number of molecules/moles are not affected by pressure.

The reaction proceeds with a decrease in number of molecules and thus decrease in pressure. Increasing pressure makes the reaction to proceed in the forward direction and produce more ammonia. When pressure is decreased, backward reaction is favored since the reaction proceeds with increase in pressure.
iii) Concentration
If the concentration of one of the substances present in equilibrium reaction is changed without change in any other conditions then, by Le Chatelier‘s principle, the position of equilibrium will move to decrease the concentration of the added substance.
Consider the reaction below occurring in a vessel,
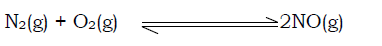
iv) Catalyst
This only increases the rate at which equilibrium is attained. It has no effect on the amount of products formed but just makes the reaction to proceed faster.
Important industrial applications of chemical equilibrium
- Synthesis of ammonia by Haber process
Ammonia is manufactured by Haber process according to the following equation:
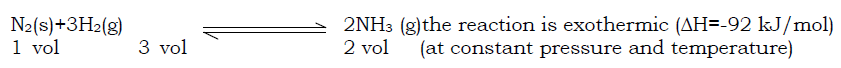
In this process, ammonia is prepared from nitrogen and hydrogen. The nitrogen used is got from distillation of liquid air and nitrogen from water gas. The yield of ammonia will depend on conditions that favour its production and these include;
High pressure: This is because the reaction proceeds with a decrease in number of moles and hence decreases in volume. High pressure would increase the yield of ammonia (Le Chatelier‘s principle), therefore a pressure of 200-500 atm is used.
Low temperature: Since the reaction proceeds with evolution of heat, the forward reaction is favoured by low temperature, so, the formation of ammonia will increase with decrease in temperature and decrease with increase in temperature. In practice a temperature of about 450˚C is used.
However, at very low temperatures, the rate of the reaction is reduced, so it is necessary to introduce a catalyst which would give a high yield in spite of a relatively low temperature. The catalyst that is used is finely divided iron.
High concentration of nitrogen and hydrogen: Increasing the concentration of either nitrogen of hydrogen leads to production of more ammonia as the added nitrogen of hydrogen will be used up to form ammonia.
- Formation of sulphuric acid by contact process
This involves the following steps
i) Formation of sulphur dioxide. The sulphur dioxide can be formed by
a) Burning sulphur in air

ii) Purification and formation of sulphur trioxide
The sulphur dioxide and oxygen are purified, mixed together and reacted to form sulphur trioxide.
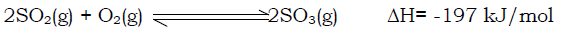
Since the reaction is exothermic, the yield of sulphur trioxide is favoured by low temperature. In practice, a temperature of 450-500˚C is used. The rate of the reaction is increased by adding a catalyst (vanadium (V) oxide).
The reaction proceeds with a decrease in number of moles and thus decrease in volume, therefore high pressure is required for more yield of sulphur trioxide. In practice, the pressure used is between 1-10 atm since the cost of maintaining high pressure is high.
iii) The sulphur trioxide is dissolved in concentrated sulphuric acid to form fuming sulphuric acid (oleum)
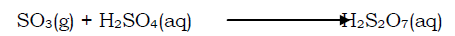
The sulphur trioxide is not directly dissolved in water because it would react exothermically.
iv) The fuming sulphuric acid is diluted with water to form very concentrated sulphuric acid (about 98% concentrated)

- Manufacture of nitric acid
Ammonia is burnt in excess air over red hot catalyst (platinum (90%)/rhodium (10%) gauze catalyst) to form nitrogen monoxide according to the reaction,


Sample questions on Rates of reaction
- Explain (i) what is meant by the term “rate of a chemical reaction” (ii) the effect of concentration of reactant on the rate of a chemical reaction. The table below shows the times taken for reaction of a certain substance Z to go to completion when solutions containing various concentrations of Z were used.

Calculate the value of 1⁄t for each time, t above and enter your answer in the space provided in the table above. Plot a graph of 1⁄t, vertical axis against concentration of Z. Deduce from your graph how the rate of the reaction varies with concentration of Z.
Draw a sketch graph to show how volume of carbon dioxide would vary with time if excess dilute hydrochloric acid was added to a certain mass Wg of marble chips and label it x. Draw on the same axes the sketch graph you would expect if equimolar volume of the hydrochloric acid was added to Wg of finely ground marble chips; and label it Y.
State one factor which can affect the rate of a chemical reaction other than concentration. Mention the effect of the factor you have stated in (d) (i) on the rate of reaction.
- Describe fully one reaction to illustrate each of the following. (a) a reaction whose rate is increased by raising temperature (b) a reaction whose rate is increased by raising pressure (c) a reaction whose rate is affected by presence or absence of light.
- The rate of decomposition of solution of hydrogen peroxide is increased by the presence of a catalyst. Write down an equation for the decomposition and name the catalyst. Describe with the aid of a diagram an experiment to measure the rate of decomposition of the hydrogen peroxide?
- Name four factors that affect the rate of a reaction. Describe how the named factors affect the rate of reaction.
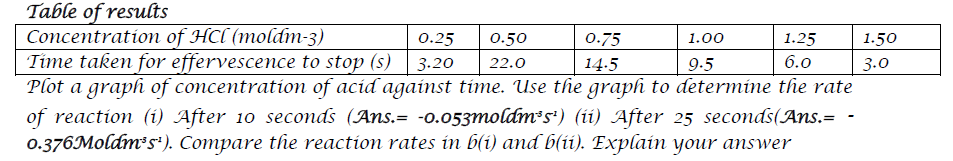
- Dilute hydrochloric acid reacts with Zinc according to the equation;

25cm3 of dilute hydrochloric acid of various concentrations were placed in six different beakers. 0.30g of the same Zinc powder were separately added to each beaker. The time taken for effervescence to stop was noted.