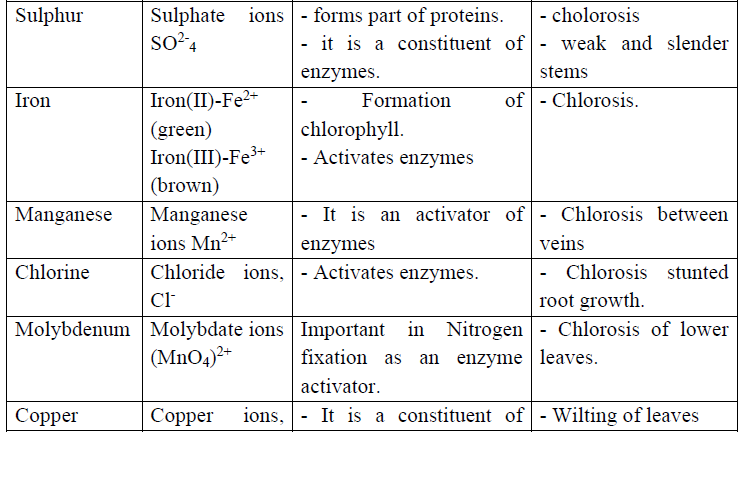
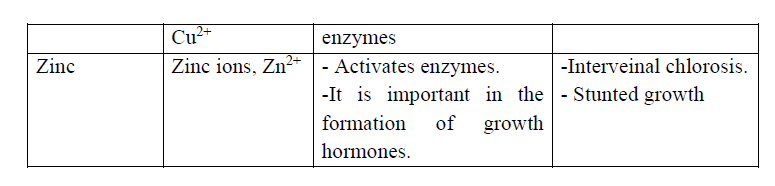
Back to: O level Biology NOTES Uganda syllabus
These are solutions with a balanced concentration of mineral salts. Such solutions are used to investigate the effect of a missing mineral element on plant life. This is done by dissolving all other minerals in water except one whose effect is being investigated.
Experimental apparatus for culture of seedlings

Precautions taken:
1) Walls of the jar should be painted white to keep light away from the culture in order to prevent the growth of unicellular algae which can bring about shortage of the minerals
2) The underside of bung should be kept dry otherwise the stem of the seedling may rote.
3) Air must be blown in through the right angled tube every day to provide oxygen for the roots
4) The solution should be renewed at the end of every two weeks.


MOVEMENT OF MATERIALS IN AND OUT OF THE CELL
Substances like nutrients and excretions move in and out of the cell by:
- Diffusion
- Osmosis
- Active transport
- Phagocytosis
- Pinocytosis
Movement of substances depends on the permeability of the cell membrane or cell wall.
DIFFUSION
This is the movement of molecules of gases and liquids from a region of high concentration to a region of low concentration. Diffusion occurs because small molecules are in constant random motion. Molecules of gases and liquids by random motion tend to distribute themselves evenly, throughout the available space, unlike in solids where molecules are closely packed together and have no freedom of movement. Diffusion only takes place where there is a difference in concentration i.e. where there is a concentration gradient and continues until there is even distribution of molecules.
EXPERIMENT TO DEMONSTRATE DIFFUSION IN GASES
Apparatus
Wet red litmus paper,
cotton wool,
glass tube,
ammonium solution,
glass rod
Method
Some strips of wet red litmus papers are stuck on the walls of a glass tube as indicated below.
The glass tube is corked as one end and a piece of cotton wool is soaked in ammonium solution and is introduced at the other end which is also plugged.
Procedure
Squares of wet red litmus paper were pushed with a glass rod or wire into a wide glass tube so that they stick to the side and are evenly spaced out. The glass tube is corked at one end the other end is closed with a cork carrying a plug of cotton wool, soaked in ammonia
Observation
The alkaline ammonia gas, diffused along the glass tube, turning the litmus papers blue in succession from 1to 5, showing that the ammonia gas was diffusing from one end to the other.
NB: If the experiment is repeated using more dilute solution of ammonia, the rate of diffusion would be seen to be slower.
EXPERIMENT TO DEMONSTRATE DIFFUSION IN LIQUIDS
Materials
Glass beaker
Potassium permanganate crystals
Water
spatula
Procedure. - Squares of wet red litmus paper were pushed with a glass rod or wire into a wide glass tube so that they stick to the side and are evenly spaced out. The glass tube is corked at one end the other end is closed with a cork carrying a plug of cotton wool, soaked in ammonia
- Observation
- The alkaline ammonia gas, diffused along the glass tube, turning the litmus papers blue in succession from 1 to 5, showing that the ammonia gas was diffusing from one end to the other.
- NB: If the experiment is repeated using more dilute solution of ammonia, the rate of diffusion would be seen to be slower.
EXPERIMENT TO DEMONSTRATE DIFFUSION IN LIQUIDS
Materials
Glass beaker
Potassium permanganate crystals
Water
spatula
Procedure
Fill a glass beaker with about 50cc of water
Place a few crystals of potassium permanganate at the base of the beaker in the water.
Leave the set up for about 30 minutes.
Observation
After 30-40 minutes, the potassium permanganate color will have spread first at the bottom and later upward to color all the water in the beaker.
Conclusion
Diffusion occurs in liquids.
FACTORS AFFECTING THE RATE OF DIFFUSION
1) Concentration gradient
Concentration gradient is the difference in concentration between the 2 regions where diffusion takes place. The higher the concentration gradient between the two regions, the faster is the rate of diffusion.
2) Temperature
The higher the temperature of the substances (molecules), the faster is the rate diffusion, because temperature increases the kinetic energy of molecules.
3) Size/density of molecules
The smaller the molecules, the faster the rate of diffusion. The denser the particle, the lower the rate of diffusion.
4) Distance over which diffusion occurs
The shorter the distance between the two regions of different concentration, the greater is the rate of diffusion like the alveoli of lungs or the epithelial linings of the ileum are thin to provide a short distance for diffusion thus increasing the rate of diffusion.
5) Surface area over which diffusion occurs
The larger the surface over which diffusion is to take place, the faster is the rate of diffusion e.g. diffusion surfaces like the ileum have numerous villi to increase the rate of diffusion.
Types of diffusion
Simple diffusion
This is the type of diffusion where molecules or ions move freely across the cell membrane without being aided.
Facilitated diffusion
This is where molecules or ions move across the cell membrane by being aided by protein carriers using energy.
Significance of diffusion to organisms
i) It helps substances to move in and out of cells.
ii) Plant root hairs take up some salts by diffusion
iii) Unicellular microorganisms like amoeba, take in oxygen and pass out carbon dioxide through the cell membrane by diffusion.
iv) Digested food e.g. simple sugars, amino acids, enter the blood from the gut by diffusion.
v) Once dissolved in blood, the food substances diffuse out of the blood into the cells where they are needed.
vi) Oxygen diffuses into blood and CO2 out of blood in the lungs of mammals and gills of fish by diffusion.
vii) Waste products of metabolisms e.g. nitrogen containing substances like urea, diffuse out of the animal cells into blood.
